Gut microbiota plays a crucial role in host health during aging.
How the gut microbiota accelerates cellular aging and the mechanisms of its effects on human aging are still unclear.
On January 10th, 2025, Chao Zhao’s team from Fudan University published an article entitled “Gut microbial-derived phenylacetylglutamine accelerates host cellular senescence” in the journal Nature Aging.
By analyzing samples from healthy individuals aged 22 to 104 years, the study found that gut microbiota derived metabolite phenylacetic acid (PAA) and its metabolite phenylacetylglutamine (PAGln) increase with age in the host.
Mechanistically, PAGln promotes cellular senescence through the ADR-AMP-activated protein kinase (AMPK) signaling pathway, leading to mitochondrial dysfunction and DNA damage.
Pagln-induced aging can be improved by ADR blocking and in vivo anti-aging therapy.
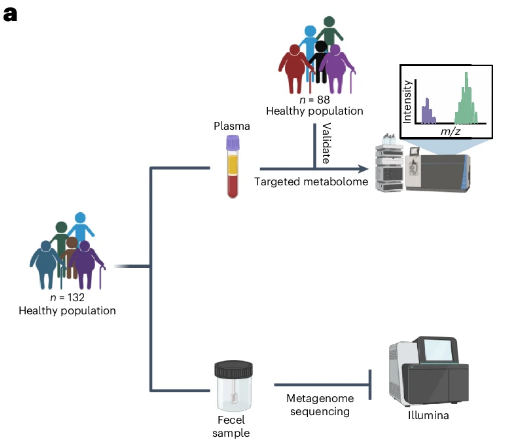
To characterize the metabolic characteristics of the body’s aging process, the researchers performed metabolome tests on samples from healthy people, including 132 individuals between the ages of 22 and 104.
A total of 166 circulating metabolites were detected in all samples.
The correlation analysis showed that phenylacetic acid metabolism was the most enriched metabolic pathway.
The gut microbiome and host co-metabolite PAGln also showed the strongest positive correlation with age.
Further recruiting the healthy cohort and referring to other omics results, the combined analysis found that PAGln increased with age in healthy people.
In primates, the gut flora can convert dietary L-phenylalanine (L-Phe) into PAA, which is then absorbed into the bloodstream and combines with glutamine in the liver or kidneys to form PAGln.
Further studies found that there is a synergistic change between plasma PAA and PAGln and gut microbiome characteristics during aging, and that the gut microbiome of the elderly has a stronger PAA production capacity.
To explore the possibility of PAGln triggering cellular senescence with increasing age in healthy people, the researchers then intervened with different concentrations of PAGln in cell models.
It was found that long-term PAGln intervention induced cellular senescence.
Further animal experiments also found that PAGln treatment can induce cellular senescence in vivo.
What Value points out is that PAA-producing bacteria can also promote host cell aging in vivo.
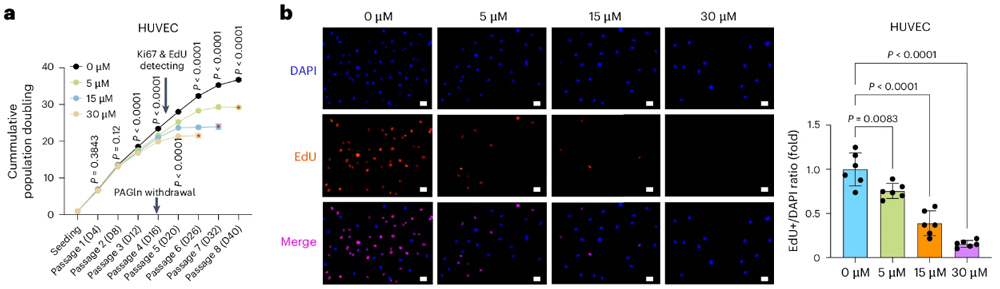
In order to explore the specific mechanism of PAGln induced cell aging, the researchers used sequencing and bioanalysis techniques, combined with a variety of other experimental means, and found that PAGln can induce mitochondrial dysfunction through the ADR-AMPK pathway, resulting in cell DNA damage.
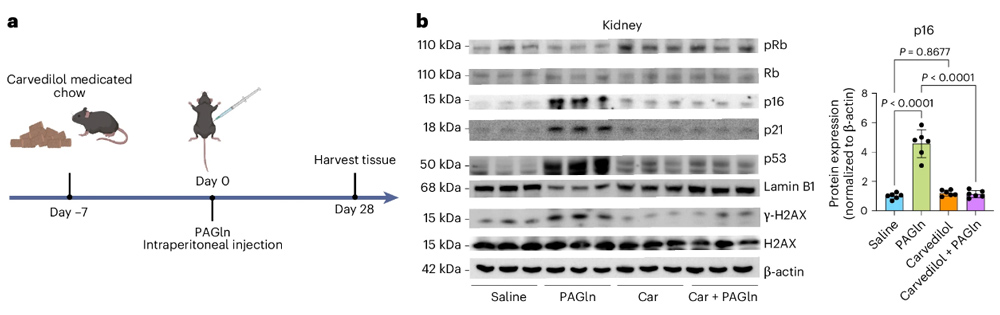
Finally, the researchers tested the ability of carvedilol, which is known to inhibit ADRs that can inhibit ADRs, to improve PAGLN-induced aging.
The results showed that PAGln induced an increase in cell aging markers, but the expression of aging markers decreased after carvedilol intervention.
Encouragingly, in vivo experiments also showed that carvedilol intervention significantly reduced the levels of aging markers in the kidneys and lungs of mice.
The anti-aging drug Bcl-2 inhibitor ABT263 is also known to significantly delay aging.
The study systematically reveals the mechanisms by which changes in the gut microbiome accelerate cell aging, both in vitro and in vivo, and provides insight into how the microbiome and host co-metabolite PAGln increase and affect the host with age.
The study also found that blocking ADR and anti-aging therapy could delay PAGLN-induced cellular aging in vivo, providing a new idea for combating aging.
References: