Abstract
Recently, Zhong Cheng/Xin Bo team of Tianjin University of Science and Technology College of Bioengineering published an article in the Journal of Agricultural and Food Chemistry: Tandem GGDEF-EAL domain proteins pleiotropically modulates c-di-GMP metabolism enrolled in bacterial cellulose Biosynthesis (https://doi.org/10.1021/acs.jafc.4c07301), which reported GGDEF – EAL tandem structure domain protein c – di – GMP metabolism and affect the performance of strains of phenotype and fermentation, The biosynthesis capacity of bacterial cellulose (BC) was enhanced by modifying the engineered bacteria for c-di-GMP metabolism.
Dr. Tianzhen Zhang, Class 2019, School of Bioengineering, Tianjin University of Science and Technology, is the first author of the paper, and Lecturer Bo Xin and Professor Cheng Zhong, Tianjin University of Science and Technology, are the co-corresponding authors.
content
Bacterial Cellulose (BC) is a kind of green bio-based polymer material, which is widely used in food, daily chemical, medicine and other fields.
Komagataeibacter xylinus is an important substrate strain for BC production due to its broad substrate spectrum and high BC synthesis capacity.
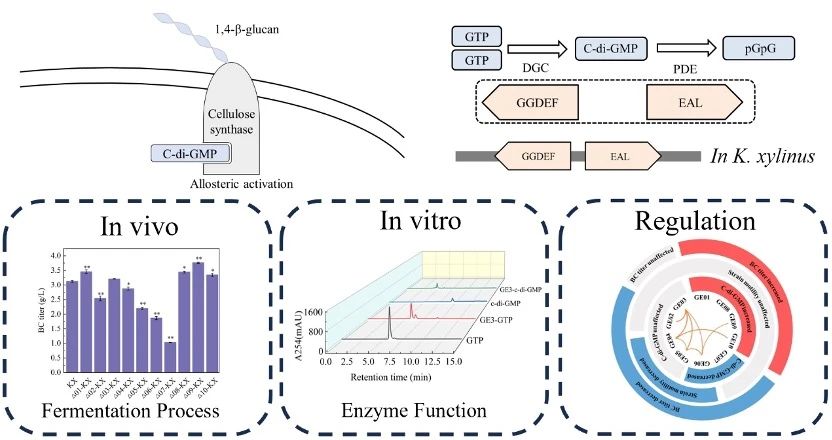
Cyclic diguanylate (c-di-GMP) is a second messenger molecule that variably activates cellulose synthase and is synthesized by Diguanylate cyclase (DGC) containing the conserved GGDEF domain. It is degraded by Phosphodiesterase (PDE) containing the EAL domain.
There are several GGDEF-EAL tandem domains in K. xylinus CGMCC 2955, and their c-di-GMP metabolic function is still unclear.
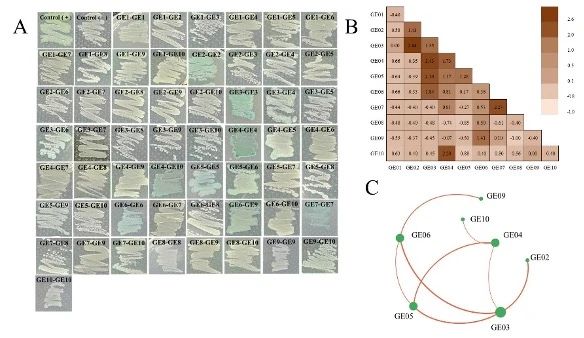
In order to clarify the mechanism of c-di-GMP regulation of BC biosynthesis, domain prediction and motif analysis of 10 GGDEF-EAL tandem domain proteins (GE01-GE10) were performed in this study.
Among them, only GE04 contained 8 transmembrane domains, GE05 found the inhibition site RXXD sequence (I site) near the upstream of the GGDEF motif, and all 10 proteins had 7 key amino acid residues “E-N-E-E-D-K-E” in the catalytic center of the EAL domain.
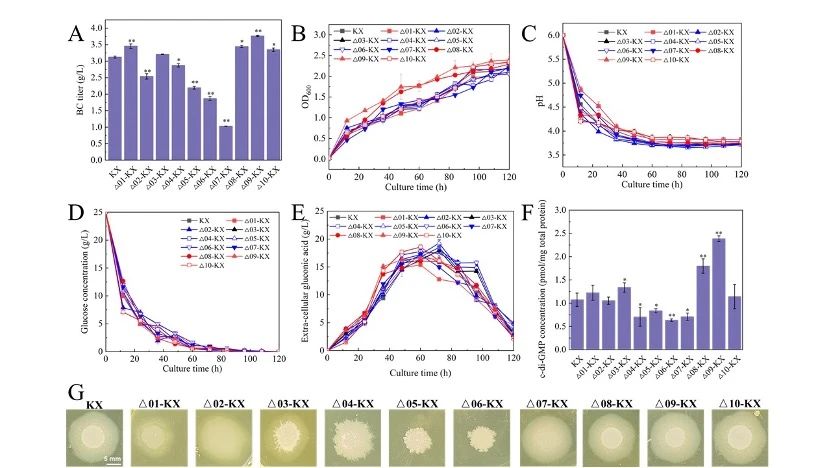
In this study, 10 GGDEF-EAL protein-deficient mutant strains were constructed and their phenotypes and fermentation performance were investigated.
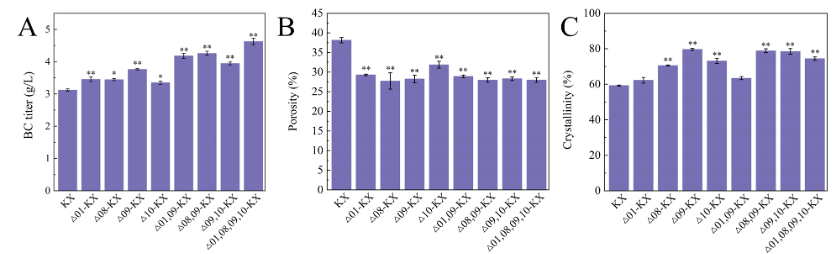
Among them, the BC production of Δ01-KX and Δ09-KX increased by 10.5% and 20.5%, respectively, and Δ08-KX and Δ10-KX increased by 10.2% and 7.2%, respectively.
The yields of Δ02-KX, Δ04-KX, Δ05-KX and Δ07-KX were slightly lower than those of the wild type.
Intracellular c-di-GMP content was positively correlated with BC production.
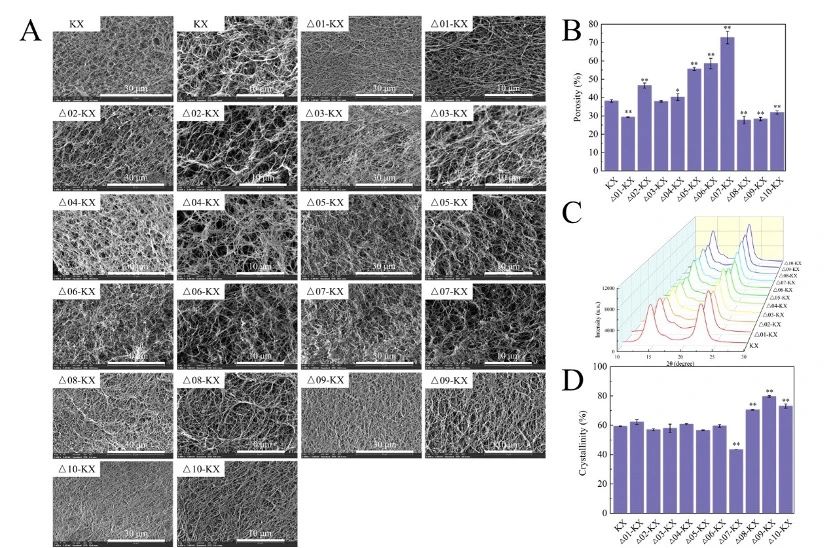
The microscale structure of BC shows that the BC produced by Δ02-KX, Δ05-KX, Δ06-KX, Δ07-KX has a more loose structure, while the BC produced by Δ01-KX, Δ08-KX, Δ09-KX, Δ10-KX has a more dense network structure.
Further, the c-di-GMP metabolic function of GGDEF-EAL protein was investigated by simulated catalytic system.
GE02, GE03, GE05, GE06 and GE07 can synthesize c-di-GMP, while GE01, GE03, GE08, GE09 and GE10 can degrade c-di-GMP.
GE03 has a unique dual function of c-di-GMP synthesis and degradation.
In addition, the interaction ability between GGDEF-EAL proteins and protein domains was explored based on bacterial bilecolecular hybridization.
The results show that GE03, GE04, GE05 and GE06 constitute the interaction center network, and the GGDEF domains of DGC have various interaction capabilities.
GE03 is a protein with multiple interaction capabilities, each of which interacts with one or more other protein domains.
Finally, based on the modification method that can promote the synthesis of BC, the engineered strains with the combination of knockout GE01, GE08, GE09 and GE10 were constructed. The results showed that the BC yield of δ01,08,09, 10-kx reached 4.62 g/L, which was 48% higher than that of the wild type.
In this study, GGDEF-EAL series domain proteins of BC production chassis K. xylinus were systematically studied, which deepened the understanding of c-di-GMP signal transduction and its regulation mechanism of BC synthesis, and provided effective strategies to improve the efficiency of BC production.
The research was supported by the National Natural Science Foundation of China, the Disruptive Innovation Project of Haihe Laboratory of Synthetic Biology, and the National Key Research and Development Plan.