01 Abstract
Following the work on constructing the highest L-threonine yield from polyploid Escherichia coli [LiangQuanfeng Shanshan/Qi Qingsheng group Adv Sci | Created artificial polyploid Escherichia coli and applied to threonine synthesis] published in the leading journal Advanced Science, Liang Quanfeng/Qi Qingsheng team of the State Key Laboratory of Microbial Technology of Shandong University published an online paper entitled “Combinatorial metabolic engineering of Escherichia” on January 10, 2025 in Bioresource Technology coli to efficiently produce L-threonine from untreated cane molasses “.
This study focused on the development of a L-threonine producing E. coli chassis. The engineered strain was able to use glucose as a carbon source to achieve L-threonine production with high yield (154.2 g/L), high conversion (0.76 g/g) and high production intensity (2.14 g/L/h).
An engineered strain (92.5 g/L) was constructed to produce L-threonine from inferior biomass sugarcane molasses, and the substrate cost of L-threonine production was reduced by 48%.
02 Content
L-threonine is widely used in food, pharmaceuticals and cosmetics, mainly as a feed additive.
Although several metabolic engineering strategies have been developed to enhance L-threonine production in engineered microorganisms, the cost of producing L-threonine remains a limiting factor for the widespread use of L-threonine in downstream products.
In this study, a combination of metabolic engineering methods, including optimization of key genes in L-threonine biosynthesis pathway, optimization of L-threonine transport system, Global transcription mechanical engineering (gTME) screening of transcription factor mutants to improve L-threonine production and utilization of poor biomass sugarcane molasses, was used to achieve the development of efficient, economical and environmentally friendly engineered strains.
The results of this study provide a potential method for producing L-threonine from agricultural waste, thereby reducing the overall cost of L-threonine synthesis.
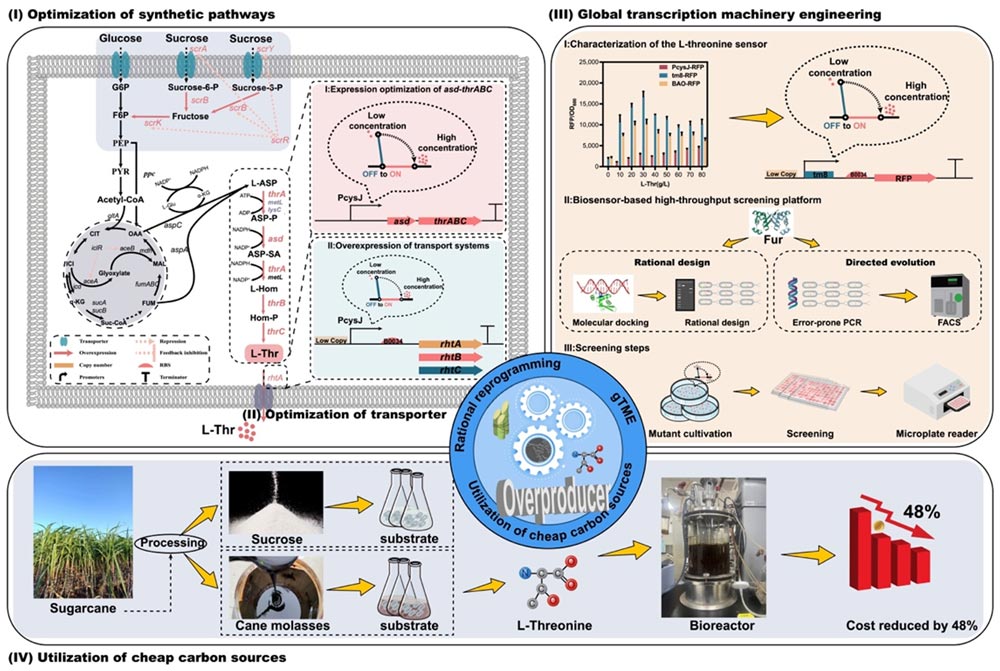
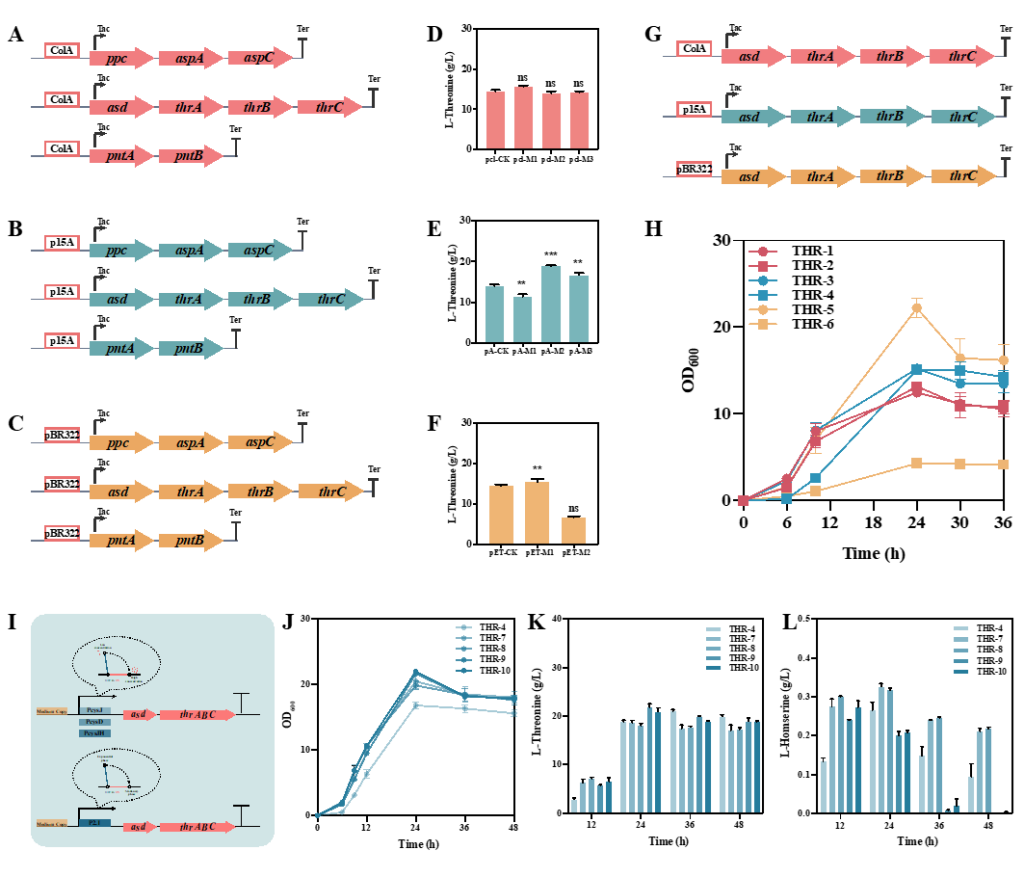
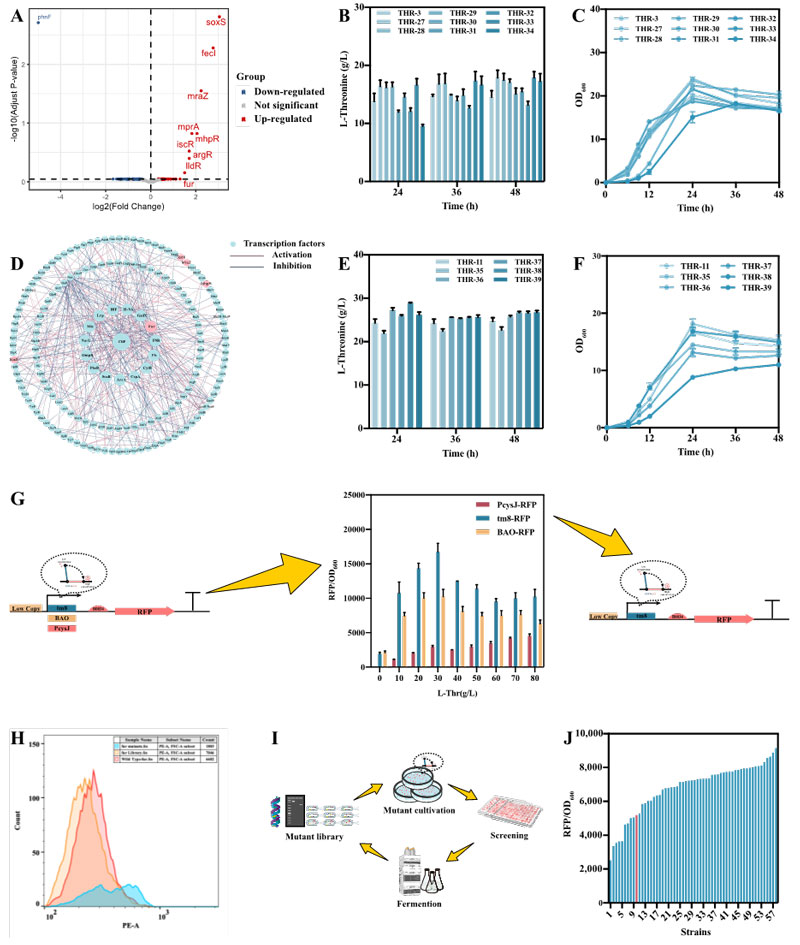
Firstly, modular metabolic engineering strategy was used to determine the optimal expression level of key genes of L-threonine biosynthesis pathway. L-threonine production was increased by 34.0% in medium copy number plasmid expression of asd and thrABC, but the growth was poor due to metabolic burden.
Therefore, the production of L-threonine reached 21.78 g/L in 24 h and the fermentation cycle was shortened by using the stable activated promoter P2.1 and L-threonine in response to the dynamic regulation of promoters PcysD, PcysJ and PcysJH.
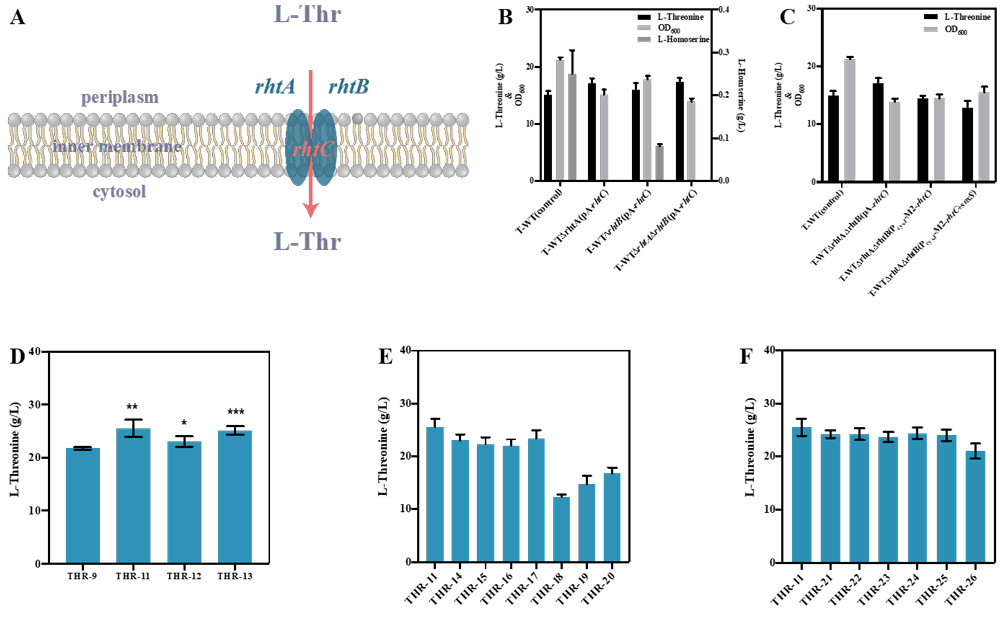
On this basis, L-threonine response activated promoter PcysJ was used to control the expression of rhtA, rhtB and rhtC. After rhtA expression, L-threonine production was increased by 17.3%.
The expression of rhtA was further optimized by promoter engineering and RBS engineering, but the L-threonine production was not further improved.
The dynamic regulation of rhtA is more effective for the production of L-threonine, and the optimal rhtA expression L-threonine yield is 25.51 g/L.
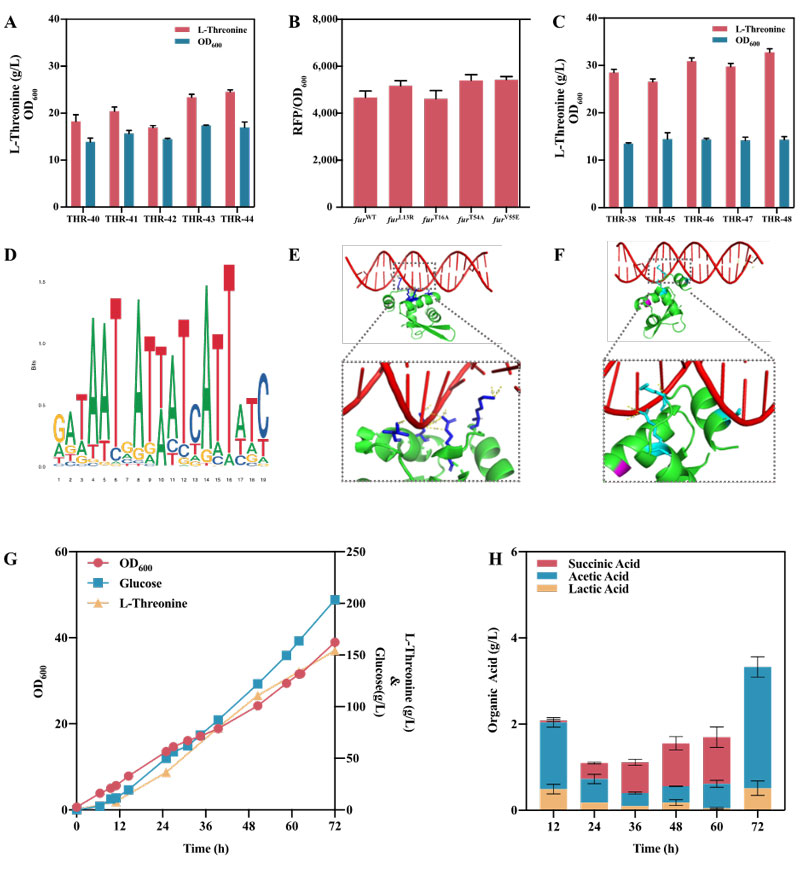
Then, a new transcription factor Fur associated with L-threonine biosynthesis was identified. Two strategies of directed evolution and rational design were used to create a Fur mutant library. The Fur mutants were sorted by flow cytometry (FACS) using L-threonine biosensors.
The FurV55E mutant obtained the highest L-threonine yield of 32.8 g/L, which reached 154.2 g/L in fed-batch fermentation in a 5 L bioreactor, comparable to the previously reported maximum yield of 160.3 g/L, but more competitive in terms of both conversion and production intensity.
Finally, a saccharose E. coli was constructed to produce L-threonine in a 5 L bioreactor with sugarcane molasses as the substrate, and L-threonine yield reached 92.5 g/L and 154.2 g/L when glucose was used.
Feedstock cost analysis shows that the production cost of sugarcane molasses is 48% lower than that of glucose.
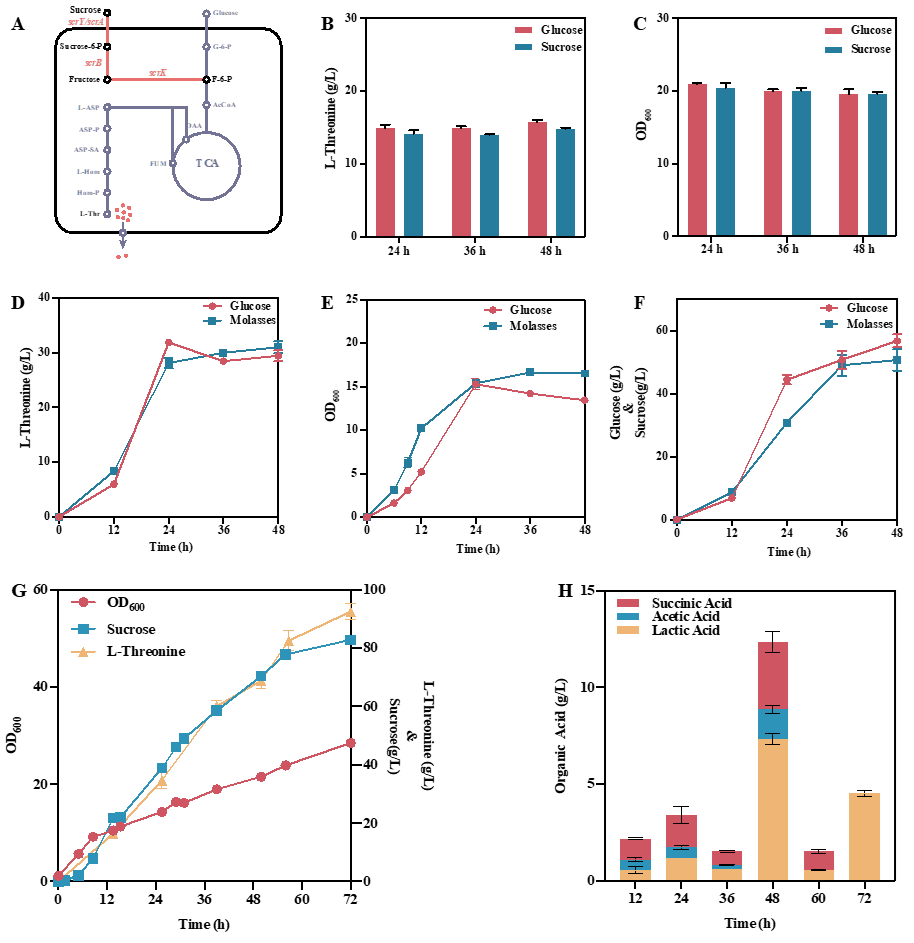
Jin Xin, PhD candidate from the State Key Laboratory of Microbial Technology of Shandong University, is the first author of the paper, and Professors Liang Quanfeng and Qi Qingsheng from Shandong University are corresponding authors.
The research was supported by the National Key Research and Development Program (2022YFC3401300) and the National Natural Science Foundation of China (32470065, 31770095).
Reference materials:
- Wang, S., Chen, X., Jin, X., Gu, F., Jiang, W., Qi, Q., Liang, Q. 2023a. Creating Polyploid Escherichia Coli and Its Application in Efficient L-Threonine Production. Adv Sci (Weinh).
- Diesveld, R., Tietze, N., Furst, O., Reth, A., Bathe, B., Sahm, H., Eggeling, L. 2009. Activity of exporters of Escherichia coli in Corynebacterium glutamicum, and their use to increase L-threonine production. J Mol Microbiol Biotechnol. 16, 3-4, 198-207.
- Dong, X., Quinn, P.J., Wang, X. 2012. Microbial metabolic engineering for L-threonine production. Subcell Biochem. 64, 283-302.