Oral liposome glutathione: An exploration of absorptive safety and detoxification effect
introduction
In the field of nutritional supplements, the absorption of vitamins and dietary supplements has always been a hot topic.
Vitamin C, for example, although it plays an important role in maintaining human health, its bioavailability gradually decreases as the dose is consumed, especially at doses above 250 mg, most of which cannot be effectively absorbed, thus limiting its potential in disease prevention and treatment.
Liposomes, as a new drug delivery system, provide a new way to solve this problem.
Liposomes can protect the active ingredients they contain from the destruction of stomach acid and digestive enzymes, and promote the absorption of nutrients through a variety of mechanisms, thereby increasing their concentration in the blood and giving full play to their physiological functions.
The application of liposome technology in the field of drug delivery has made significant progress, and many pharmaceutical companies utilize synthetic liposome formulations to improve the efficacy of drugs.
In the field of nutritional supplements, some innovative supplement companies have also begun to use natural liposome preparations to deliver a variety of low-bioavailable nutrients, such as vitamins C, D, B complex, mineral magnesium, natural products curcumin and resveratrol, as well as important cell antidotes and free radical scavengers glutathione.
These liposomal preparations can not only improve the absorption rate of nutrients, but also provide additional nutritional support for the body’s vital organs and cells.

Characteristics of liposomes
Liposomes are submicroscopic bubbles composed of lipids, especially phosphatidylcholine.
These bubbles can be loaded with a variety of bioactive substances and efficiently delivered into the bloodstream, rather than just being excreted through the digestive tract.
The size and distribution of liposomes are critical to their performance.
Studies have shown that the size of the liposomes is evenly distributed and compact, with 95% of the liposomes between 50 and 420 nanometers in size.
In addition, liposomes remain stable at room temperature for up to 18 months, making them an ideal drug delivery system.
The size of the liposomes is large enough to carry the payload while small enough to provide efficient absorption, which provides a unique advantage for the delivery of nutrients.
Liposomes first protect the payload from the harsh acidic environment in the stomach during delivery.
Once through the stomach, the payload is absorbed through one of the many mechanisms available to the liposomes and delivered to where it is needed.
The higher absorption rate of this nutrient or botanic medicine allows it to achieve its full benefits.
Another advantage of liposomes is that they increase the cycle time of the payload, ensuring that every part of the body gets the nutrients it needs.
Natural and effective liposome system quality
- It is a low-viscosity fluid that is easy to pour out.
- Opaque, because it’s a gel.
- The main ingredient must be water.
- Ingredients should be natural, not hydrogenated or made from synthetic chemicals or high concentrations of alcohol.
Observation effect of liposome glutathione
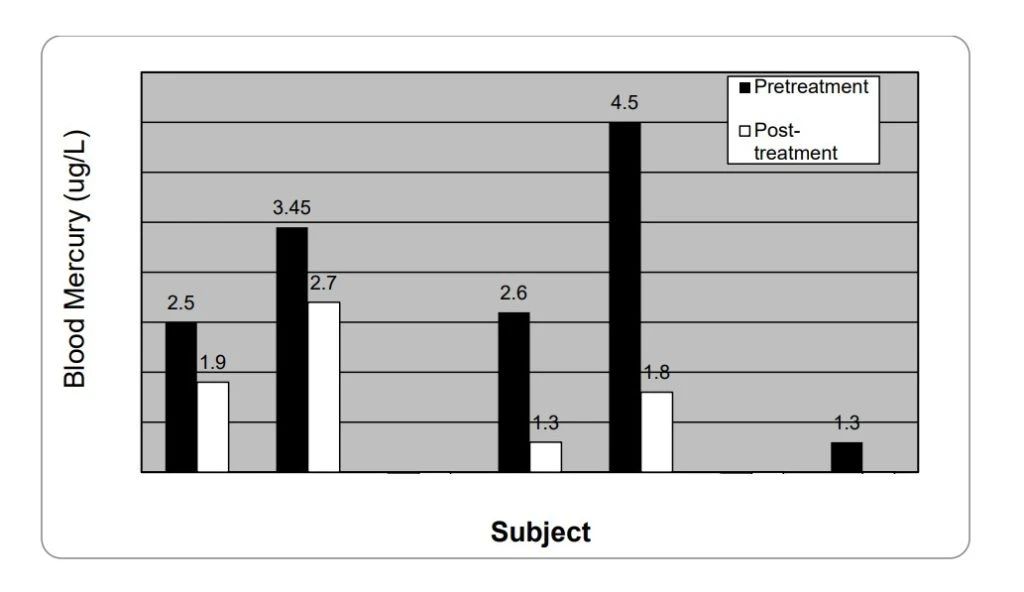
Above are the blood mercury levels of seven adults before (black) and after (gray) liposomal glutathione supplementation for 28 days.
Blood mercury levels in subject 2 were below the limit of quantification (1μg /L Hg) at day 28, while subjects 3 and 6 were below detectable levels before and after treatment.
Note: Data provided by the Energy Medical Institute, Boulder, Colorado.
Detoxification effect of liposome glutathione
In daily life, people are inevitably exposed to various toxins and poisons, such as mercury.
Mercury exposure comes from a wide range of sources, including coal power generation, mercury use in energy-efficient light bulbs, amalgam dental fillings, mercury-containing foods (such as certain fish), and vaccination.
These exposure routes lead to the accumulation of mercury in the human body, posing a potential health threat.
Traditionally, glutathione precursors such as n-acetylcysteine have been used to provide the body with the raw material needed to increase glutathione in order to enhance the body’s ability to detoxify.
However, direct measurement of glutathione levels in the blood is currently difficult.
As a result, some studies have begun to look at the effects of liposome glutathione on mercury excretion in the blood.
In a four-week study, seven subjects had their blood mercury, bilirubin and creatinine levels monitored before and after supplementation with liposomal glutathione.
The results showed that liposomal glutathione supplementation reduced blood mercury levels by at least 8% within a week, and after four weeks, five subjects had significantly lower blood mercury levels, with improvements ranging from 19% to 60%, while the other two had blood mercury levels below the detection limit both before and after treatment.

This suggests that liposome glutathione can effectively promote the operation of the second and third stages of the human detoxification system.
In a small 30-day open-label study, subjects who consumed 750 mg of liposomal glutathione liquid twice daily had an average 39% reduction in blood mercury levels, along with improvements in blood bilirubin and creatinine levels, suggesting that liposomal glutathione has a positive role in promoting heavy metal detoxification as well as protecting liver and kidney function.
Glutathione is essential for organ function, and increasing glutathione levels in the subjects not only enhanced their detoxification ability, but also improved liver and kidney function.
This is manifested by reduced levels of bilirubin and creatinine in the blood.
Notably, even if some subjects’ blood mercury levels did not change significantly, their bilirubin and creatinine levels still improved, further confirming the multifaceted benefits of liposomal glutathione in maintaining body health.
conclusion
Glutathione, as a key endogenous antioxidant, plays a vital role in maintaining normal physiological functions of the body.
However, traditional oral glutathione absorption is inefficient, limiting its effectiveness in clinical applications.
In recent years, the development of liposome technology provides a new way to improve the bioavailability of glutathione.
The aim of this study was to investigate the absorptive safety of oral liposome glutathione and its potential effect on detoxification.
By comparing the absorption of standard glutathione powder with liposome glutathione liquid (CELLg8®) in the human body, and monitoring the effects of liposome glutathione on blood mercury, bilirubin and creatinine levels, it was found that liposome formulation significantly increased blood glutathione concentrations by an average of 20 times. It is proved that liposome glutathione has significant advantages in improving absorption efficiency, enhancing detoxification effect and maintaining liver and kidney function.
Liposome technology provides an efficient and safe new way for the delivery of nutritional supplements, and is expected to play a greater role in future clinical applications, bringing people a healthier lifestyle and a more personalized nutritional supplement program.
With the deepening of research, the potential application of liposomal glutathione will be more extensive, and its value in disease prevention, treatment and health promotion will be further explored and utilized.
reference
- 1. Groff, J.L., Gropper S.S., and Hunt S.M. The Water Soluble Vitamins. In: Advanced Nutrition and Human Metabolism. Minneapolis: West Publishing Company, 1995, p. 222-237
- 2. Keller BC. Liposomes in nutrition. Trends Food Sci & Tech. 2001; 12: 25-31.
- 3. Liposomal amphotericin B (Gilead Sciences), Liposomal doxorubicin (Zeneus), Liposomal vaccines (Berna biotech)
- 4. Wotschi A, Reddy S, Stofer B, Lauterburg BH. The Systemic Availability of Oral Glutathione. Eur J Clin Pharm. 1992; 43:667-669.
- 5. Torchilin VP, Recent Advances with Liposomes as Pharmaceutical Carriers. Nature Reviews, Drug Discovery 2003; 4: 145-160.
- 6. Clakson TW, Vyas JB, Ballatori N. Mechanisms of Mercury Disposition in the Body. Am J Indus Med. 2007; 50:757-764.
- 7. Molin M, Schutz A, Skerfving S, Sallsten G. Mobilized mercury in subjects with varying exposure to elemental mercury vapour Int. Arch. Occup. Env. Health. 1991; 63: 187-192.
- 8. James SJ, Slikker W III, Melnyk S, New E, Pogribna M, Jernigan S. Thimerosal neurotoxicity in associated with glutathione depletion: Protection with glutathione precursors. NeuroToxicology. 2005; 26: 1-8.
- 9. Ross EA, Koo LC, Moberly J.B. Low whole blood and erythrocyte levels of glutathione in hemodialysis and peritoneal dialysis. Am. J. Kidney Dis. 1997; 30: 489-494.
- 10. Santangelo F, Witko-Sarsat V, Drueke T. Descamps-Latscha B. Restoring glutathione as a therapeutic strategy in chronic kidney disease. Nephrol. Dial. Transp. 2004; 19: 1951-1955.