An important index to detect the level of cellular oxidative stress is the ratio of GSH (reduced glutathione) and GSSG (oxidized glutathione) in cells. The higher the ratio of GSSG to GSH, the higher the degree of cellular oxidation.
How to detect this indicator and the detection principle?
Basic knowledge
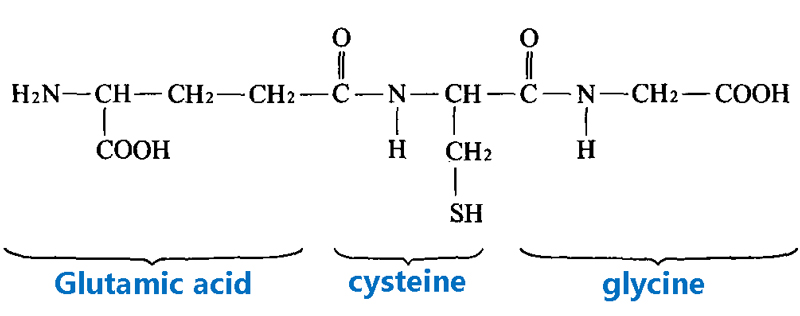
Glutathione is a small peptide composed of three amino acid residues, the full name is glutamyl-cysteinyl-glycine, the English name is Glutamyl-Cysteinyl-Glycine, short for Glutathione.
Since the sulfhydryl group (SH) on cysteine is the active group of glutathione, it is often shortened to G-SH or GSH.
There are two forms of glutathione: reduced glutathione (often called GSH) and oxidized glutathione disulfide.
Since oxidized glutathione is produced by dehydrogenation of two GSHS by sulfhydryl groups, it is often abbreviated as G-S-S-G or GSSG.
Reduced glutathione is the main source of sulfhydryl groups in most living cells, plays an important role in maintaining the proper REDOX state of sulfhydryl groups in proteins, and is a key antioxidant in animal cells.
Typically 90-95% of total glutathione is reduced glutathione.
GSSG is reduced to GSH by glutathione reductase, and GSH can react with color producing substrate DTNB to produce yellow TNB and GSSG.

When the reaction system is properly formulated and the two reactions are combined, the total glutathione (GSSG+GSH) is equivalent to a rate-limiting factor for color production, and the amount of total glutathione determines the amount of yellow TNB formation.
The amount of total glutathione can be calculated by measuring A412.
The content of GSSG can be determined by removing GSH from the sample with appropriate reagents, and then using the reaction principle mentioned above.
The amount of GSSG can be calculated by subtracting the amount of GSSG from the amount of total glutathione (GSSG+GSH).
The specific reaction principle of this kit is as follows:
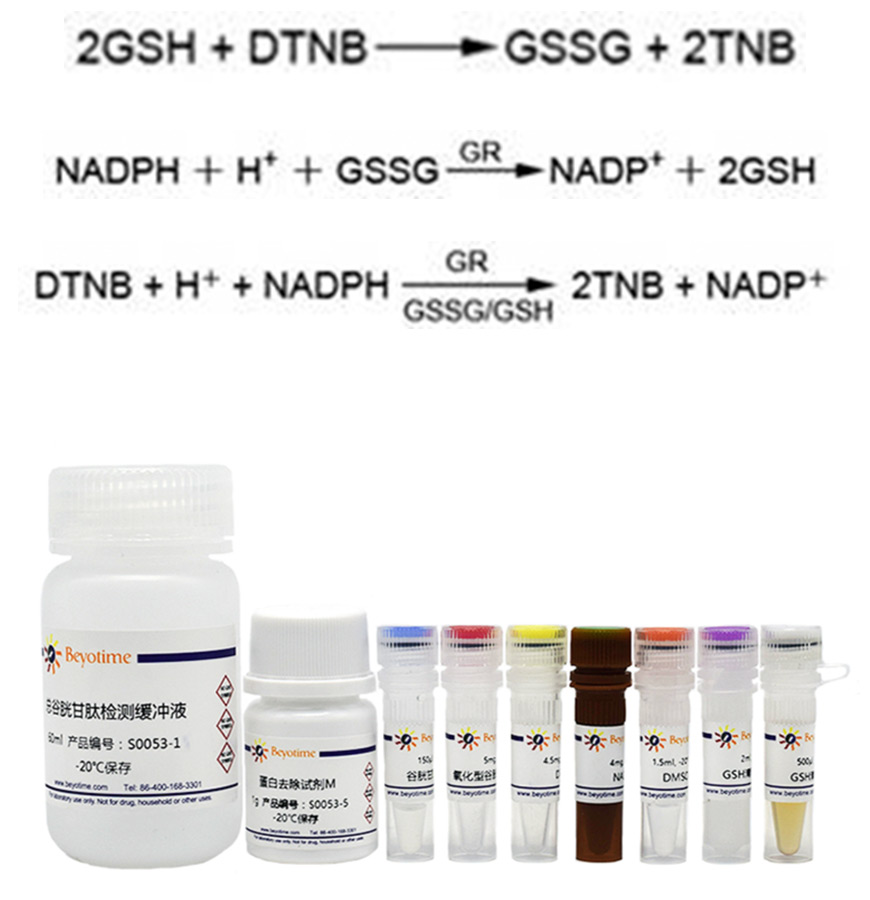
Preliminary preparation
- Reagent: super clean table in advance of pancreatic enzyme; The kit is rewarmed at room temperature 1-2 hours in advance; Centrifugal tubes (15mL or 5mL, 1.5mL, 500μL, 200μL), liquid nitrogen.
- Instrument: water bath 37℃, centrifuge pre-cooling.
Kit reagent configuration and packaging
1. Configure protein removal reagent M:
Prepare 15mL or 5mL centrifuge tube and mark tube M. After weighing 0.2g M, add 4mL total glutathione detection buffer, and prepare immediately.
The first few experiments need to determine the standard curve in a specific environment such as laboratory temperature, and more M is required. In the case of no subsequent standard curve, the number of final detection holes is determined, and each hole needs 50μL.
2. Configure GSSG reserve liquid:
Marker tube:
Four 500 µL EP tubes — GSSG reserve fluid
1 1.5mL EP tube – 15μM GSSG standard
Six 500μL EP tubes – 0, 0.5, 1, 2, 5, 10μM
14 200μL EP tubes – 0, 0.5, 1, 2, 5, 10, 15μM (two sets of standard: one total glutathione, one GSSG)
GSSG reserve solution (10mM) : 816μL ddH2O was added to 5mg GSSG. Store at -20℃ after packaging.
3. GSSG Standard:
- 15μM: 1.5μL GSSG Stock solution(10mM)+998.5μL M
- 10μM: 200μL GSSG(15μM)+100μL M
- 5μM: 100μL GSSG(10μM)+100μL M
- 2μM: 100μL GSSG(5μM)+150μL M
- 1μM: 100μL GSSG(2μM)+100μL M
- 0.5μM: 100μL GSSG(1μM)+100μL M
Add 50μL of each prepared GSSG standard to the corresponding 200μL EP tube, and add 50μL M of 0μM.
4. Diluted GSH removal auxiliary solution:
47μL ddH2O+53μL GSH removal auxiliary solution. Use now and match now.
5. Diluted GSH cleaning fluid:
89.2μL anhydrous ethanol +10.8μL GSH cleaning solution. Use now and match now.
6. Total glutathione test solution:
Per well (5 times diluted glutathione reductase – 6.6μL+DTNB reserve – 6.6μL+ total glutathione detection buffer 150μL).
- (1) 5-fold dilution of glutathione reductase: 50μL reductase +200μL total glutathione detection buffer.
- (2) DTNB: 4.5mg DTNB+1.5mL DMSO. Store at -20℃ after packaging.
- (3) Total glutathione detection buffer. 150μL per well.
7. NADPH:
(1) Reserve liquid (40mg/mL) : 4mg+100μL ddH2O. Store at -80℃ after packaging.
(2) Detection solution (0.5mg/mL) : 10μL reserve solution +790μL total glutathione detection buffer. Each well needs 50μL.
Cell sample preparation
- 1.5mL EP tube label tube, weighing. Two sets of 200μL EP tubes (labeled with GSSG and total glutathione, respectively).
- Discard the culture medium in the petri dish and clean it with PBS. Digestion of pancreatic enzymes was performed at 1200 rpm and centrifuged at 4℃ for 4min.
- After discarding the supernatant, wash it once with PBS, at 1200 rpm, 4℃, centrifuge for 4min.
- Absorb the liquid as much as possible, weigh it again, calculate the cell precipitation weight, calculated as 1mg is 1μL, the added M volume is the obtained cell weight ×3. Vortex dissolution.
- Freeze and thaw twice in liquid nitrogen and 37℃ water bath.
- Place on ice for 5 minutes.
- Centrifuge 10000g at 4℃ for 10min. Add 50μL supernatant to 200μL EP tube, the rest can be stored at -80℃ (recommended for immediate use).
Note: The concentration of the sample can be diluted in advance here. It can be diluted 5 times first for pre-test and diluted with M solution. In the experiment measured by the author, the total glutathione needs to be diluted more times and the GSSG content is low, so it is not necessary to dilute too many times.
GSH is cleared before GSSG detection
- 50μL standard and sample +10μL diluted cleaning assistant, vortex.
- Add 2μL GSH to remove the working liquid and vortex.
- Incubate at room temperature for 60min.
Total glutathione was detected
- 96-well plate, add 10μL standard and sample, add 150μL total glutathione test solution. Incubate at room temperature for 5min.
- Add 50μL 0.5mg/mL NADPH solution.
- Immediately detect A412 with enzyme marker. Test every 5 minutes.
reference
- Hu, L., Chen, L., Yang, G., Li, L., Sun, H., Chang, Y., Tu, Q., Wu, M., & Wang, H. (2011). HBx sensitizes cells to oxidative stress-induced apoptosis by accelerating the loss of Mcl-1 protein via caspase-3 cascade. Molecular cancer, 10, 43. https://doi.org/10.1186/1476-4598-10-43
- Rui, T., Wang, H., Li, Q., Cheng, Y., Gao, Y., Fang, X., Ma, X., Chen, G., Gao, C., Gu, Z., Song, S., Zhang, J., Wang, C., Wang, Z., Wang, T., Zhang, M., Min, J., Chen, X., Tao, L., Wang, F., … Luo, C. (2021). Deletion of ferritin H in neurons counteracts the protective effect of melatonin against traumatic brain injury-induced ferroptosis. Journal of pineal research, 70(2), e12704. https://doi.org/10.1111/jpi.12704