Ischemic injury is the number one cause of death and disease in the world, including the United States and China, and vascular endothelial growth factor (VEGF) gene therapy holds promise for patients with ischemic injury.
However, the high immunogenicity of traditional viral vectors (such as adeno-associated virus AAV) and the low expression efficiency of lipid nanoparticles (LNP) have been bottlenecks in VEGF delivery, leading to the failure of several previous clinical trials.
Recently, a new type of gene therapy based on engineered Extracellular vesicles (EVs) has been shown to be able to address barriers to AAV and LNP:
Compared with AAV and LNP carrying VEGF gene loading, cell-engineered exovesicles loaded with vascular endothelial growth factor A (VEGF-A) messenger RNA (mRNA) exhibited lower immunogenicity and effective delivery.
Co-corresponding authors are Dr. Andrew Lee of Shenzhen Bay Laboratory/Guangdong-Hong Kong-Macao Greater Bay Area International Clinical Trial Center and Shenzhen Graduate School of Peking University;
Dr. Patricia K. Nguyen of Stanford University School of Medicine; Dr. LAN Feng of Fuwai Hospital, Chinese Medical University; And Prof. Zhenya Shen of the First Affiliated Hospital of Soochow University and the Institute of Cardiovascular Science;
The first authors are You Yi and Tian Yu, PhD candidates at Peking University Shenzhen Graduate School, and the above researchers published a paper in the European Heart Journal on January 20, 2025 (IF: 38, ranked first in the field of cardiovascular medicine) published this research results, marking the cardiovascular disease gene therapy has entered a new era.
Breaking the bottleneck: VEGF-A cell engineering of external vesicles provides a new way to deliver genes to diseased tissues
In the process of gene therapy exploration, how to efficiently and safely deliver therapeutic genes to target tissues has been a great challenge for researchers.
Traditional delivery methods, such as adeno-associated viruses (AAV) and lipid nanoparticles (LNP), have achieved delivery of nucleic acids (such as DNA and mRNA), but their inherent immunogenicity can limit therapeutic effectiveness and may even trigger a severe immune response that not only destroys gene therapy loading, but also produces side effects for patients receiving these treatments.
In order to break through this bottleneck, the research team took a new approach and turned their attention to the engineered external vesicles, a natural nanoscale delivery carrier.
As a kind of tiny vesicles secreted by cells, engineered exovesicles have excellent biocompatibility and low immunogenicity, and can easily cross a variety of biological barriers to accurately deliver the carried genetic material to the target cell.
The research team previously developed the first dermatologic mRNA therapy, collagen replacement therapy with mRNA delivery by extracellular vesicles (EVs) (You Y et al., Nat Biomed Eng 2023). In the continuation study, full-length vascular endothelial growth factor A (VEGF-A) mRNA was successfully wrapped in the engineered external vesicles derived from fibroblasts, and VEGF-A engineered external vesicles with high mRNA content were prepared.
This innovative delivery method not only achieves efficient delivery of VEGF-A mRNA, but also significantly reduces immunogenicity, bringing a new solution to the field of gene therapy.
Angiogenesis: VEGF-A engineered engineered external vesicles promote tissue repair and regeneration
In the treatment of ischemic vascular disease, angiogenesis plays a crucial role, aiming to restore blood perfusion to the tissue and improve its function.
VEGF-A (Vascular endothelial growth factor -A), as a crucial vascular growth factor, has shown remarkable effect in inducing angiogenesis.
It can effectively promote the proliferation and migration of vascular endothelial cells and lay a foundation for the formation of new blood vessels.
However, there are obvious limitations of traditional VEGF-A protein injection or gene therapy, such as short half-life and insufficient targeting, which greatly limits its efficacy in practical applications.
This study proposes a new idea of using VEGF-A engineered external vesicles for treatment.
By delivering exogenous VEGF-A mRNA to ischemic tissue, the team significantly increased VEGF-A protein expression levels.
In mouse models in which human VEGF-A engineered external vesicles were administered after femoral artery or coronary artery ligation, the team observed significantly enhanced angiogenesis.
These new vessels are not only increased in number, but also more mature in structure, effectively improving the blood perfusion status of ischemic tissue.
At the same time, VEGF-A engineered external vesicles also show the potential to promote left ventricular function recovery, opening up a new way for the treatment of ischemic heart disease.
Low immunogenicity: VEGF-A engineered engineered external vesicles lead to a new model of gene therapy
In the process of gene therapy, immunogenicity is a problem that cannot be ignored.
Traditional AAV and LNP delivery methods often trigger a strong immune response, resulting in limited therapeutic effectiveness.
In contrast, VEGF-A engineered external vesicles, with their naturally low immunogenicity, did not elicit significant innate or adaptive immune responses at the injection site or systemic.
By comparing the immune responses of three different delivery modes of VEGF-A AAV, VEGF-A LNP and VEGF-A engineered external vesicles in mouse models, this study found that VEGF-A AAV can induce strong innate and adaptive immune responses. However, VEGF-A LNP mainly induced strong innate immune response and low adaptive immune response.
In contrast, delivery of VEGF-A engineered external vesicles caused only a low innate immune response and a low adaptive immune response, demonstrating extremely high safety and tolerability.
This finding not only provides strong evidence for the application of VEGF-A engineered external vesicles in gene therapy, but also provides new ideas for the design and optimization of other gene therapy vectors.
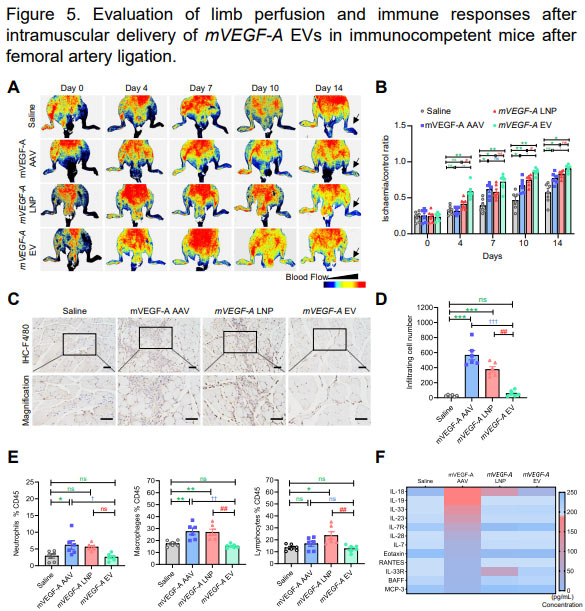
However, the revascularization of mVEGF-A EV mRNA was faster, possibly due to the lower immunogenicity shown in the C-F diagram.
Repeated administration: VEGF-A engineered engineered external vesicles demonstrated a durable therapeutic effect
Repeated administration is a common strategy in the treatment of disease, with the aim of enhancing the therapeutic effect by increasing the duration and dose of drug exposure.
However, this practice may also carry potential risks, such as in AAV-mediated gene therapy, where repeated administration can lead to increased immunogenicity and increased toxicity.
Therefore, it is particularly important to develop a new drug delivery method that can effectively enhance the therapeutic effect and avoid the above risks.
In response to this challenge, we investigated the sequential delivery effect of VEGF-A engineered external vesicles in injured skin.
The results showed that this mode of administration could not only significantly improve the wound healing effect, but also no significant immune response or toxic reaction was observed.
This suggests that, compared with conventional drugs, VEGF-A engineered external vesicles exhibit high efficiency and safety at a single dose.
More importantly, during repeated administration, VEGF-A engineered external vesicles maintained a stable therapeutic effect while exhibiting low immunogenicity.
This finding offers broad prospects for the application of VEGF-A engineered external vesicles in chronic ischemic vascular diseases and diseases requiring long-term treatment.
With the advancement of science and technology and the deepening of research, gene therapy is gradually becoming an important means to treat a variety of diseases.
However, the problems of immunogenicity and targeting faced by traditional delivery methods have been the key factors restricting their development.
Through innovative use of engineered external vesicles as delivery carriers, this study successfully prepared highly efficient and low immunogenicity VEGF-A engineered external vesicles cell engineered external vesicles therapy, bringing new hope for the treatment of ischemic vascular diseases such as heart disease, stroke and limb ischemia.
In the future, with the continuous deepening of research and continuous improvement of technology, it is believed that this new type of gene therapy will enter clinical trials and show its unique advantages and potential other therapeutic indications.
References:
- 1、Yi You, Yu Tian, Rui Guo, Junfeng Shi, Kwang Joo Kwak, Yuhao Tong, Andreanne Poppy Estania, Wei-Hsiang Hsu, Yutong Liu, Shijun Hu, Jianhong Cao, Liqun Yang, Rui Bai, Pufeng Huang, Ly James Lee, Wen Jiang, Betty Y S Kim, Shuhong Ma, Xujie Liu, Zhenya Shen, Feng Lan, Patricia Kim Phuong Nguyen, Andrew S Lee. Extracellular vesicle-mediated VEGF-A mRNA delivery rescues ischaemic injury with low immunogenicity. Eur Heart J. 2025 Jan 20:ehae883. doi: 10.1093/eurheartj/ehae883.
- 2、You Y#, Tian Y#, Yang Z#, Shi J, Kwak KJ, Tong Y, Estania AP, Cao J, Hsu WH, Liu Y, Chiang CL, Schrank BR, Huntoon K, Lee D, Li Z, Zhao Y, Zhang H, Gallup TD, Ha J, Dong S, Li X, Wang Y, Lu WJ, Bahrani E, Lee LJ, Teng L, Jiang W, Lan F, Kim BYS, Lee AS*. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat Biomed Eng. 2023 Jul;7(7):887-900. doi: 10.1038/s41551-022-00989-w. Epub 2023 Jan 12. PMID: 36635419.