Radiotherapy is a common means of treating cancer in clinical practice. It works by irradiating cancer cells with radiation to destroy their DNA and cause them to die. NMN
However, radiation can also damage a patient’s healthy cells, which are more sensitive to radiation-induced DNA damage because of the working environment in which gut cells must be kept up with rapid renewal.
For example, 60%-80% of patients who have undergone radiotherapy for abdominal and pelvic cancer will experience early intestinal damage symptoms such as abdominal pain, acute diarrhea, and nausea, 2 which are the side effects of radiotherapy.
A protein called NRF2 plays a key role in the body’s response to the side effects of radiation therapy, regulating how the body fights oxidative stress and helps repair damaged DNA.
However, as people age, the level of NRF2 protein will gradually decline, so it is more difficult for the elderly to cope with the intestinal damage caused by radiation.
However, researchers at Peking Union Medical College have found that oral NMN (β-Nicotinamide Mononucleotide) can protect mice with defective NRF2 protein expression from intestinal damage caused by radiotherapy, which means that NMN is expected to help elderly cancer patients resist the side effects of radiotherapy on the stomach and intestines.
The study was published in October 2022 in the journal Free Radical Biology and Medicine.
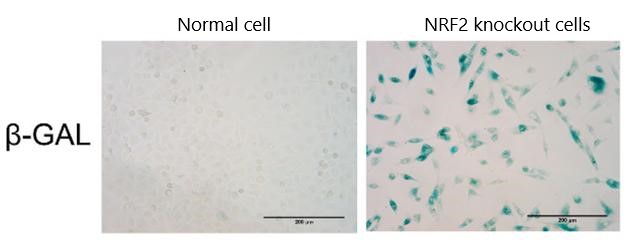
The researchers first performed in vitro cell culture experiments to determine the effects of radiation on intestinal cells. To simulate the situation of elderly patients, the researchers also used gene editing to knock out the gene that expresses NRF2 in mouse cells (NRF2 knock-out).
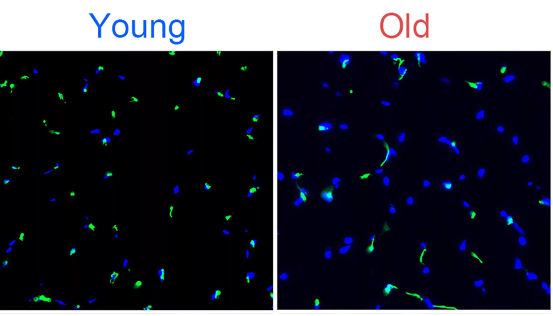
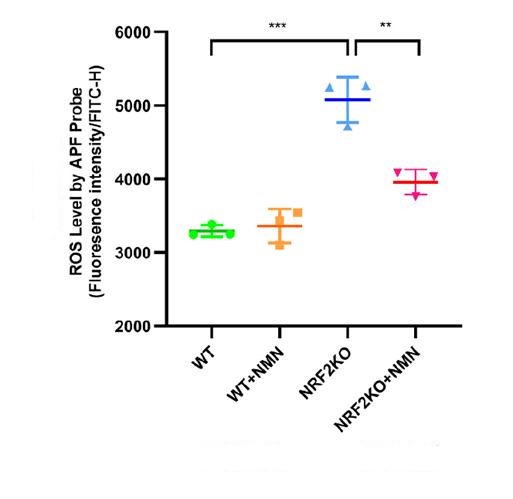
The results showed that the intestinal epithelial cells of mice lacking NRF2 protection received radiation irradiation, the level of reactive oxygen species (ROS) in the cells significantly increased, and the cells accelerated aging.
However, if the cells received 48 hours of NMN supplementation (1mmol/L) followed by radiation exposure, the levels of oxygen free radicals in these cells were significantly reduced.
The researchers then conducted animal experiments in mice to assess the effect of NMN on gut protection in vivo.
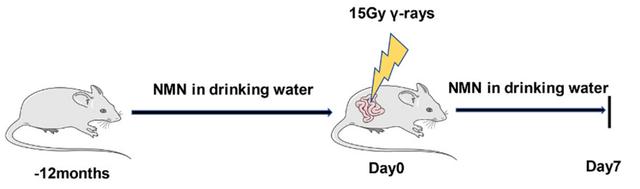
Male mice aged 8 weeks (roughly equivalent to 17 years in humans) were given 300mg/kg of NMN orally daily for 12 months.
The mice were then exposed to 15Gy radiation in the abdomen for seven days. Some of these mice were also gene-edited to knock out the NrF2-related gene to mimic the decline in NRF2 in aging.
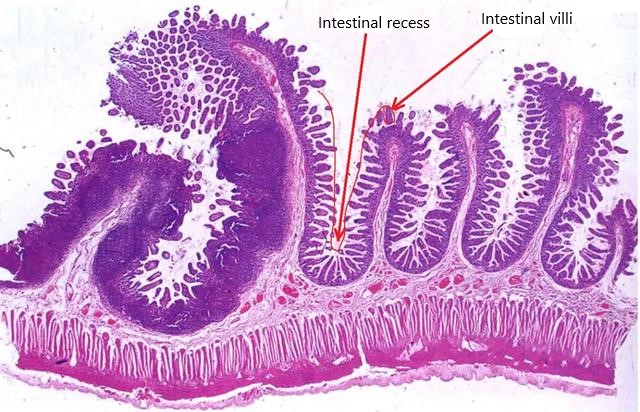
To assess the damage caused by radiation to the mice’s intestines, the researchers used an electron microscope to measure the length of villi and the depth of intestinal crypts in the mice’s intestinal tissue.
In general, the longer the villi in the gut and the deeper the recesses, the greater the surface area that can be used to digest and absorb food, and the better the health of the gut.
The results showed that the length of villi in the intestine and the depth of intestinal recess were significantly reduced after radiation exposure in simulated senescent mice with NRF2 gene knocked out, while the supplementation of NMN reversed this situation, demonstrating that NMN indeed has the effect of protecting the intestinal tract of mice from radiation damage.
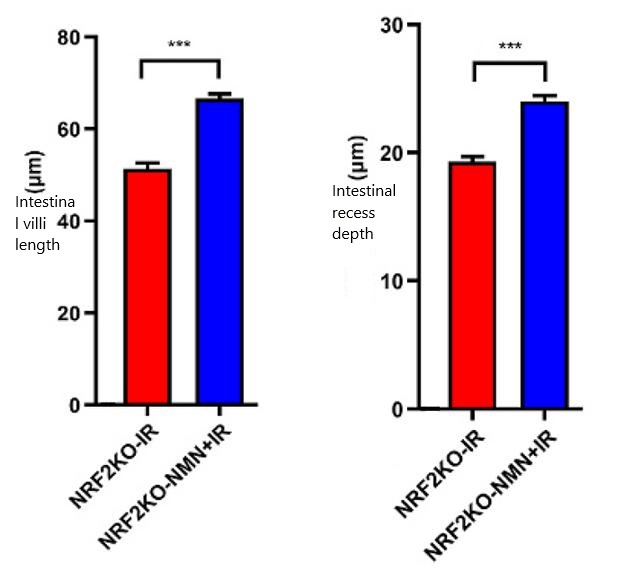
In summary, this study found that NMN can protect mice from intestinal damage caused by radiation when the level of NRF2 protein is reduced.
Since the NRF2 protein in the elderly is also reduced, NMN may be one of the means to prevent the side effects of radiation therapy in elderly cancer patients.
reference
- 1. Zhao, X., Zhang, M., Wang, J., Ji, K., Wang, Y., Sun, X., Xu, C., Wang, Q., He, N., Song, H., Du, L., Wang, F., Huang, H., Liu, Y., & Liu, Q. (2022). NMN ameliorated radiation induced damage in NRF2-deficient cell and mice via regulating SIRT6 and SIRT7. Free radical biology & medicine, 193(Pt 1), 342–353.
- 2. Hauer-Jensen, M., Denham, J. W., & Andreyev, H. J. (2014). Radiation enteropathy–pathogenesis, treatment and prevention. Nature reviews. Gastroenterology & hepatology, 11(8), 470–479.