Research into the use of bacteria in cancer treatment dates back to the 1860s.
Due to the difficulty in ensuring the safety, controllability and efficacy of bacterial therapy at that time, coupled with the emergence and widespread clinical application of more directly killing tumor therapies such as radiotherapy and chemotherapy, research on bacterial tumor therapy has stalled in the following decades.
Several key questions have long puzzled scientists:
How do bacteria evade the innate immune system?
How can bacteria stimulate anti-tumor immunity only within tumors?
How to ensure the safety of bacterial therapy?
These problems have become the bottleneck of bacterial therapy towards clinical application.
In recent years, the rapid development of synthetic biology has given birth to a variety of new anti-tumor bacteria, which has opened up a new research direction in the field of tumor immunotherapy.
Further elucidation of how these different effects coexist and systematic analysis of the molecular mechanism of the interaction between bacteria and the host immune system by using synthetic biotechnology-modified bacteria are not only the theoretical basis for promoting the rational design of synthetic bacteria, but also an important prerequisite for fully releasing its potential as a living drug therapy.
On March 3, 2025, Liu Chenli, a researcher at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences and director of the National Key Laboratory of Quantitative Synthetic Biology, led the team.
In conjunction with a team led by Xiao Yizhuan, a researcher at the Shanghai Institute of Nutrition and Health of the Chinese Academy of Sciences, the paper was published online in Cell journal with the title “Bacterial immunotherapy leveraging IL-10R hysteresis for both phagocytosis. evasion and tumor immunity revitalization “.
In this study, quantitative synthetic biology was applied to the study of bacterial treatment of tumor, using synthetic bacteria with targeted colonization and anti-tumor effects to quantitatively analyze the key factors and interaction mechanisms between bacteria and tumor.
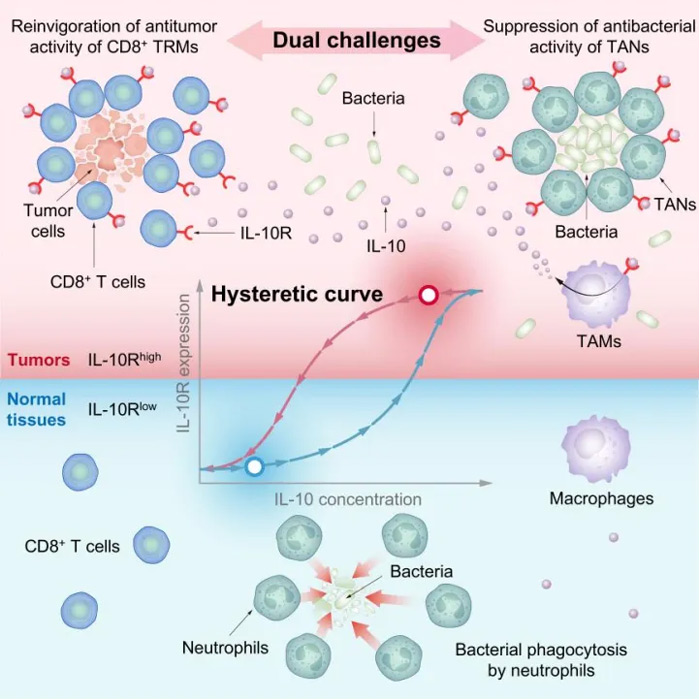
By building mathematical models and combining them with a series of quantitative experiments, they found that the bacteria took advantage of the high expression of the interleukin-10 receptor (IL-10R) in immune cells within tumors and the low expression of immune cells in normal tissues to achieve the triple goal of “targeting solid tumors, avoiding the innate immune system, and killing cancer cells.”
The discovery of this single mechanism provides important guidance for the rational design of a new generation of synthetic bacterial therapies, and is a vivid demonstration of quantitative synthetic biology in the field of biomedicine.
The research team first used Salmonella as chassis cells to construct a synthetic strain that could efficiently survive and proliferate in tumor tissue, while being quickly cleared in normal tissue.
They found that synthetic bacteria have good therapeutic effects in animal models of colon cancer, melanoma, bladder cancer and other diseases.
Then, after screening for a variety of key cytokines, the research team found that interleukin-10 (IL-10) is essential for bacteria to play a role in the treatment of tumors.
Through further quantitative studies, the research team found that IL-10R is highly expressed on a variety of immune cells such as CD8+ T cells, macrophages, and neutrophils in tumors, and proved that this property is indispensable for immune cells in tumors to play their respective roles under the mediation of bacteria.
In contrast, bacteria interact with immune cells in normal tissues differently than they do in tumors because IL-10R levels are low, and the bacteria are quickly cleared by neutrophils.
Combined with mathematical models and quantitative experiments, the team revealed a “hysteric effect” on the expression of IL-10R on the surface of immune cells, that is, when immune cells receive stimulation with high concentration of IL-10, IL-10 activates STAT3 through IL-10R, and STAT3 activates IL-10R expression in combination with the IL-10R promoter.
Thus, a positive feedback loop of IL-10/IL-10R is formed, and the nonlinear “hysteresis effect” characteristic will promote the high expression of IL-10R in cells. Even if the concentration of external IL-10 is reduced, the expression of IL-10R can still be maintained at a high level, forming a memory effect.
Combined with the temporary rise and then fall of IL-10 during tumor formation, the discovery of the “hysteric effect” for the first time revealed the molecular principle behind the high expression of IL-10R in immune cells within tumors.
Taking advantage of the high expression of IL-10R in immune cells within tumors, synthetic bacteria can stimulate macrophages within tumors to produce more IL-10 by binding to Toll-like receptor 4 (TLR4).
These newly generated IL-10 can activate a type of immune cell in the tumor that was originally “sleeping” – tumor tissoon-resident memory CD8+ T cells (CD8+ TRM cells), so that it can restore the ability to kill tumor cells.
At the same time, IL-10 can reduce the motility of tumor-associated neutrophils (TANs) in the process of bacterial treatment of tumors, thus slowing their clearance of bacteria in the tumor.
At the same time, the team found that the bacteria did not target the tumor through chemotaxis, as traditionally thought, but rather caused the tumor to “target” through differentiated growth in the tumor and normal tissue.
These results demonstrate a key mechanism by which bacteria target solid tumors while simultaneously “protecting themselves” (avoiding immunity) and “killing the enemy” (killing the tumor).
Thus, it answers the key scientific question of “why bacteria can activate ‘anti-tumor immunity’ while avoiding ‘antibacterial immunity'”, which has long puzzled the field of bacterial treatment of cancer.
In 2024, Liu Chenli and Zhao Guoping explained the research paradigm and discipline connotation of “quantitative synthetic biology” for the first time in Nature Review:
Bioengineering, and this work using synthetic bacteria to find quantitative rules vividly demonstrates the characteristics and potential of the second research paradigm of quantitative synthetic biology.
In the process of “synthetic” bacteria treating tumor, there emerged “unexpected functions” that “both want and want”, and this is the opportunity for quantitative synthetic biology to discover new principles of bacterial treatment of tumor.
Once the principles are understood, more complex synthetic systems can be designed based on these principles, further advancing the life sciences.
Reference: https://doi.org/10.1016/j.cell.2025.02.002