Exploring Cy-Dual probes: An innovative mechanism of glutathione and tyrosinase co-activation in melanoma diagnosis
Source journal impact factor and author’s academic background
- Journal information: Dyes and Pigments (2023 Impact factor: 4.7, JCR Q1), focusing on dyes, pigments and their applications in biomedicine, materials science and other fields.
- Corresponding author: Li-Qiong Yao (Department of Laboratory Medicine, The First Hospital of Lanzhou University). Her research interests include molecular diagnosis, biomedical imaging and tumor marker detection.
- Other authors: The team members are from the First Hospital of Lanzhou University and the First Clinical College of Medicine, and their research areas focus on fluorescence probe development, tumor molecular imaging, and precision medicine.
Research background
Melanoma is a highly aggressive malignancy with a survival rate of less than 10% in advanced patients, while early diagnosis can significantly improve prognosis through surgery (80% of patients are recurrence-free 10 years after surgery).
However, traditional diagnostic methods (such as histochemical tests) cannot achieve real-time monitoring in vivo, which limits the development of precision diagnosis and treatment.
Near infrared (NIR) fluorescence imaging technology has become an important tool for real-time tumor monitoring due to its advantages of high sensitivity and low tissue spontaneous fluorescence interference.
At present, most NIR probes only respond to a single biomarker (such as tyrosinase TYR), but TYR is also expressed in some normal melanocytes, leading to the risk of false positives.
Warburg effect leads to excessive reactive oxygen species (ROS) in melanoma cells, which requires high concentration of glutathione (GSH) to maintain REDOX balance, and glutathione can participate in melanin synthesis by regulating TYR activity.
Based on the synergistic effect of TYR and Glutathione in melanoma, the development of double-locked NIR probes (which need to activate fluorescence in response to both markers) has become the key to improve diagnostic specificity.
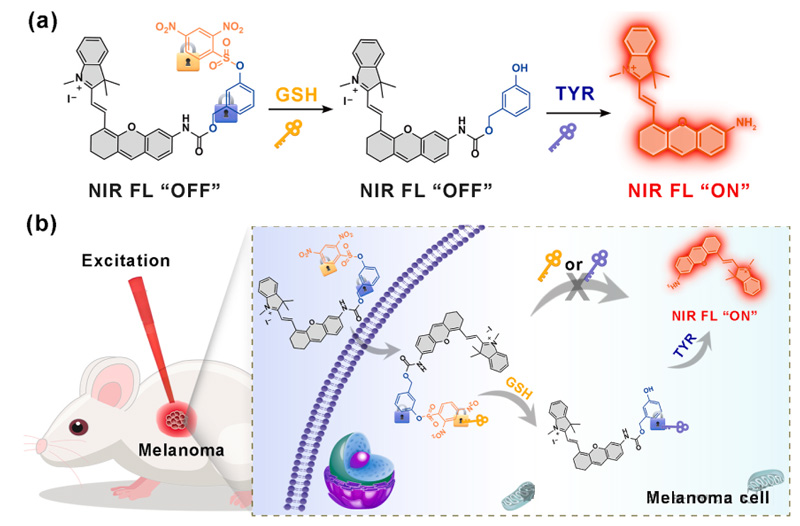
In this paper, a Cy-Dual NIR probe co-activated by glutathione and TYR is proposed for the first time, aiming to achieve accurate imaging of melanoma through a double-locked design.
Full text summary
In this paper, we designed and validated a novel double-locked NIR fluorescent probe Cy-Dual, which achieves melanoma specific detection through the synergistic activation of Glutathione and TYR.
The probe core is a semi-cyanine dye (CyN), which establishes a double locking mechanism by introducing a TYR response group and shielding its enzyme active site (activated by Glutathione).
In normal cells, Cy-Dual fluorescence was in the “off” state.
However, in melanoma cells, Glutathione first unshields through nucleophilic substitution reaction, and then TYR hydrolysis releases CyN, activating NIR fluorescence (λem=705 nm).
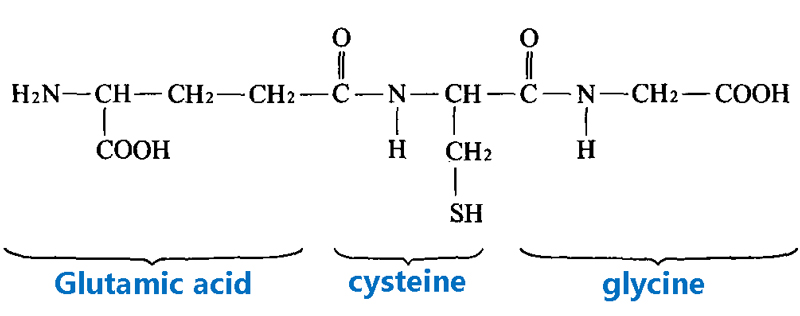
Main contents:
Probe design and synthesis: 2, 4-dinitrobenzene sulfonyl chloride was combined with 3-hydroxyl benzyl alcohol through esterification reaction to form a double response group, and then coupled with CyN to obtain Cy-Dual.
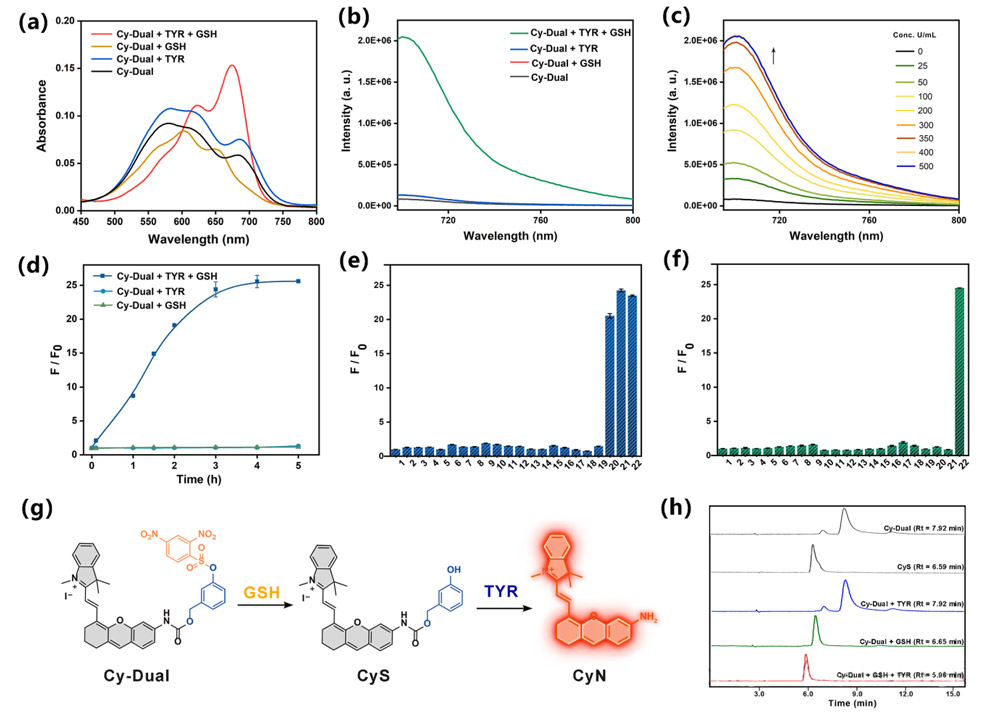
Spectral verification: Cy-Dual only showed absorption peak redshift (660 nm) and fluorescence emission (705 nm) when Glutathione and TYR co-existed, and did not respond to single-factor treatment.
Cell imaging: Significant NIR signaling was observed in A375 melanoma cells, while no fluorescence activation was observed in HeLa, HepG2 (high Glutathione but low TYR) and normal LO2 cells.
In vivo imaging: In the tumor-bearing mouse model, the fluorescence intensity of Cy-Dual at the tumor site peaked at 18 hours, and imaging of isolated organs showed that probe metabolism was mainly excreted through the liver and kidney.
Biocompatibility: MTT assay and CALcin-AM /PI staining confirmed that Cy-Dual had no significant effect on cell viability (survival rate >85%).
In this study, Glutathione and TYR were used as co-activation targets for the first time, and the false positive rate was significantly reduced through the double locking mechanism, providing a new tool for accurate diagnosis and surgical navigation of melanoma.
Summary of clinical significance and thought
Clinical significance
- Accurate diagnosis: Cy-Dual avoids false positives from single marker probes through a double-locking mechanism, which can specifically distinguish melanoma from normal tissue and facilitate early diagnosis.
- Surgical navigation: The NIR fluorescence characteristics of the probe (650-750 nm) are suitable for real-time intraoperative imaging and assist doctors to accurately remove tumor boundaries.
- Efficacy monitoring: Dynamic fluorescence signals can reflect the metabolic status of the tumor, providing a visual tool for evaluating the effectiveness of targeted therapy or immunotherapy.
Research idea
- Target selection: Based on the pathological characteristics of TYR overexpression and high level of GSH in melanoma, a dual-target synergistic activation strategy was proposed.
- Molecular design: The GSH response group is chemically modified to bind to the TYR substrate to achieve “and gate” logic control (activated only when two targets coexist).
- Transformation validation: From in vitro spectroscopy, cell imaging to tumor-bearing mouse models, the specificity, sensitivity and biosafety of the probe were gradually verified.
This study provides a paradigm for the development of multi-target activation probes, which can be extended to other tumor marker combinations (such as pH/ enzyme, hypoxia/metalloproteinases) in the future, pushing molecular imaging techniques towards higher specificity.
innovativeness
- Double locking mechanism: The NIR probe co-activated by GSH and TYR was reported for the first time, which significantly improved the detection specificity through the “and gate” design and avoided the false positive problem of single-target probes.
- Pathological association: Based on the synergistic pathological mechanism of TYR and GSH in melanoma (Warburg effect and oxidative stress), the probe design closely fits the biological characteristics of the disease.
- In vivo and in vitro validation: Systematic validation of probe performance at cellular, tissue and in vivo levels, enabling real-time NIR imaging of melanoma for the first time, and identifying its metabolic pathways.
- Clinical translation potential: The probe has low toxicity, high signal-to-noise ratio (S/N>20) and long wavelength emission (705 nm), which is suitable for deep tissue penetration and in vivo application.
Compared with existing studies (such as the H₂O₂-TYR probe developed by Peng et al.), Cy-Dual further improves diagnostic accuracy through a dual locking strategy, providing a new tool for precision medicine.
limitation
- Metabolism and toxicity: Enrichment of probes in the liver and kidney may lead to long-term toxicity, and further evaluation of their metabolokinetics and chronic exposure risk is needed.
- Complex environmental adaptation: Most experiments were conducted in PBS or single cell lines, and the stability of the probe in the blood, inflammatory microenvironment, or different subtypes of melanoma (e.g. acrotype, mucosal type) was not verified.
- Interference effects: High concentrations of Cys/H₂S may activate the probe, although their intracellular concentration is low, but may affect the outcome in specific pathological conditions (e.g., cystinuria).
- Barriers to clinical translation: The pharmacokinetic properties (such as half-life, tumor targeting) of the probe need to be optimized, and the safety of the probe needs to be verified in large animal studies.
Future studies could enhance the tumor selectivity of probes in combination with targeted delivery systems, such as nanocarriers, and explore their potential in combination with therapeutic agents.
reference
Wu D, Gan C, He C, et al. Glutathione and tyrosinase Co-activated near-infrared fluorescent probes for precise detection of melanoma[J]. Dyes and Pigments, 2025, 237: 112712. doi:10.1016/j.dyepig.2025.112712