The metabolic engineering of North Chemical Engineering team reformed Saccharomyces cerevisiae to synthesize red myrrh efficiently
As a natural sesquiterpenoid compound, alpha-red myrrh has broad application prospects in medicine and cosmetics because of its unique aroma and remarkable antioxidant properties.
However, in the production process of α-red myrrh, the traditional plant extraction method has limited raw materials, low extraction efficiency and complex extraction process, which greatly increases the production cost.
In this context, microbial fermentation technology has become an ideal choice for the production of alpha-red myrrh.
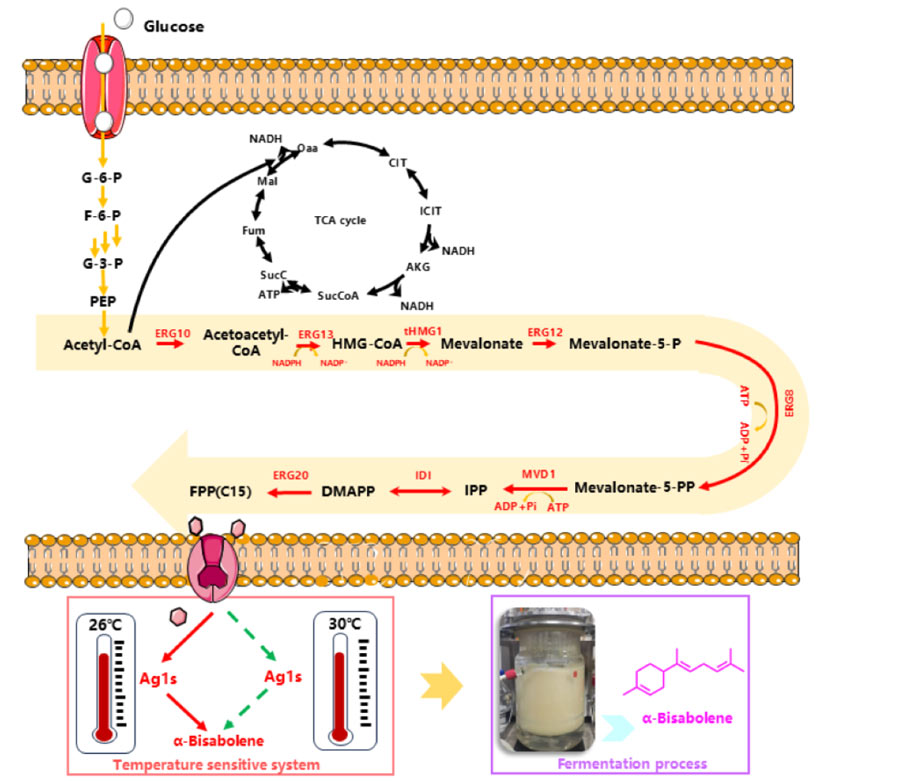
Saccharomyces cerevisiae not only has an endogenous mevalonate (MVA) metabolic pathway, it can use glucose to independently synthesize Farnesipyrophosphate (FPP), a key precursor of α-red myrrh, but also the genetic operation of saccharomyces cerevisiae is relatively simple and the fermentation process is easy to control, which provides the possibility for large-scale production of α-red myrrh.
In practice, the production of alpha-red myrrh by saccharomyces cerevisiae still faces a difficult problem, that is, it is difficult to accurately grasp the balance between cell growth and product synthesis – premature activation of the product synthesis pathway will inhibit yeast growth, while delayed start-up will reduce its production efficiency.
The metabolic burden also limits cell growth, which in turn affects alpha-red myrrh production.
To solve this problem, Professor Meng Wang of Beijing University of Chemical Technology and his team recently published a paper entitled “Developing Thermosensitive Metabolic Regulation Strategies in the ACS Synthetic Biology.
Fermentation Process of Saccharomyces cerevisiae to Enhance α-Bisabolene Production, Using a number of strategies, including enhanced precursor supply, temperature-sensitive regulation, and fermentation medium optimization, the research team successfully constructed saccharsacchara cerevisiae with a high yield of alpha-red myrrh.
Finally, the yield reached 18.6g/L in fed-batch fermentation, which is the highest yield reported to date, and provides a new approach for industrial-scale terpene biosynthesis.
In order to enhance the supply pathway of FPP, the precursor of alpha-rhodophylene production, the researchers overexpressed related endogenous genes from acetyl-CoA to FPP in the MVA pathway, including ERG10, ERG13, tHMG1, ERG12, ERG8, MVDI, IDI, and ERG20. A series of engineered strains were constructed.
The results showed that the yield of FPP increased significantly with the increase of the number of integrated genes.
BFSC0001 strain enhanced by comprehensive metabolic pathway produced 264.8 ng/mL of FPP, 44.9 times that of the initial strain 5DΔ. The titer of α-red myrrh reached 139.9mg /L after 96 h in the shaker.
However, the enhanced supply of FPP alone is not sufficient to achieve efficient production of alpha-red myrrh.
The researchers further found that alpha-red myrrh synthetase (Ag1) had limited activity and was unable to convert all FPP into alpha-red myrrh, leading to the accumulation of FPP.
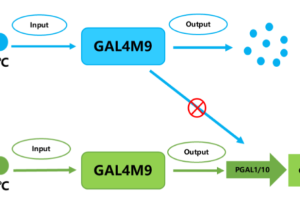
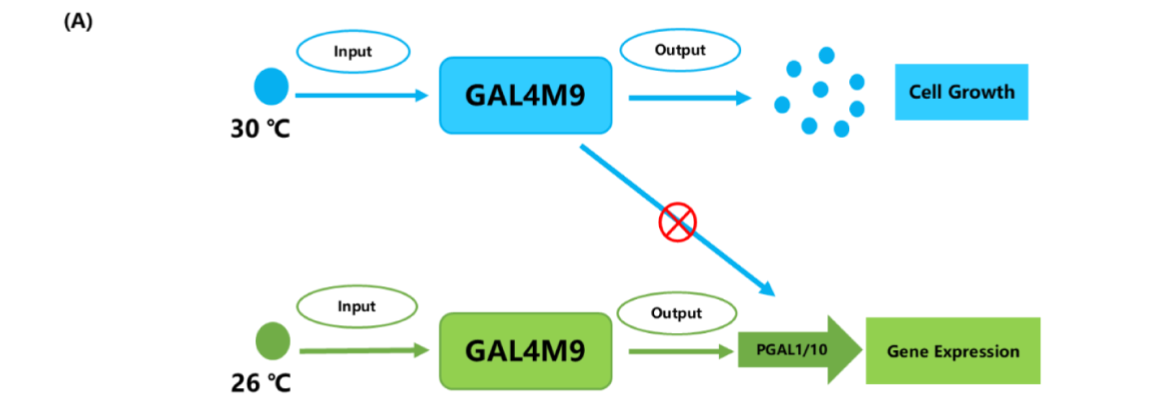
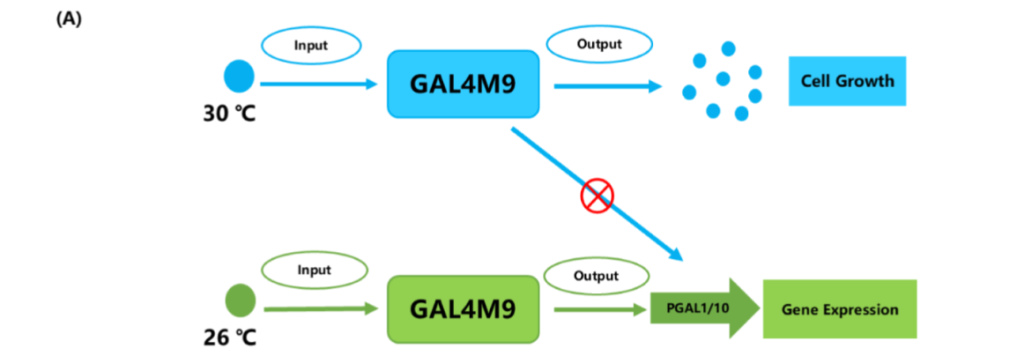
They introduced temperature-sensitive GAL4M9 to replace GAL in regulating Ag1 expression, thereby achieving temperature-dependent gene expression.
At higher temperatures (such as 30 ° C), cells can fully grow and accumulate enough biomass;
At lower temperatures, such as 26 ° C, the expression of the target gene is activated, which promotes the synthesis of alpha-red myrrh.
This two-stage fermentation strategy not only reduces the negative effects of intermediate metabolites on cell growth, but also significantly reduces the metabolic burden, and realizes the double increase of biomass and product accumulation.
In order to improve the yield of α-red myrrh, the team optimized the promoter, and finally determined that the pCYC1 promoter performed best under the two-stage fermentation condition of 30℃/26℃, so that the yield of α-red myrrh reached 216 mg/L, which was 56.5% higher than that of the single starting strain fermented at 30℃.
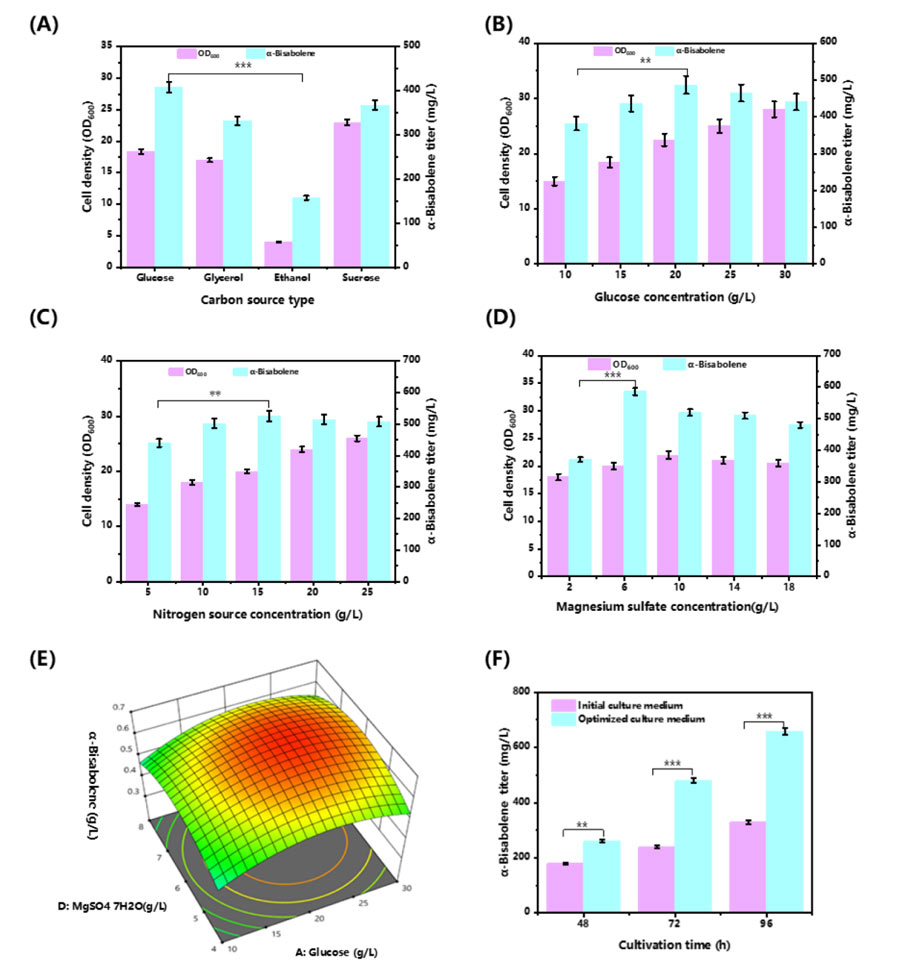
To further optimize the fermentation production of alpha-red myrrh, the researchers also relied on metabolomics analysis.
By adding different concentrations of amino acids to the fermentation medium, they found that the addition of exogenous amino acids such as arginine, methionine, glutamic acid, serine and aspartic acid could significantly promote bacterial growth and increase the yield of α-red myrrh.
In particular, when 100 mg/L of calcium D-pantothenate was added, the production of alpha-red myrrh increased by 55.6%.
This is because calcium D-pantothenate, as A precursor of Coenzyme A, plays a key role in the synthesis of acetyl-CoA, a key precursor for the synthesis of alpha-red myrrh.
Through response surface model analysis, the researchers also determined the optimal fermentation medium composition, including 20 g/L glucose, 15 g/L ammonium sulfate, and 6.25 g/L magnesium sulfate heptahydrate.
Under these optimized conditions, the yield of α-red borrh reached 658 mg/L in shake-flask fermentation, which was 98.3% higher than that of the unoptimized basic fermentation medium.
In order to verify the application effect of the constructed α-rhodoborescene Saccharomycete platform strain in actual industrial production, the researchers conducted fed-batch fermentation experiments in 2.5L and 30L bioreactors.
By precisely controlling parameters such as pH value and stirring speed during the fermentation process, the 18.6 g/L yield of alpha-red myrrh was finally achieved in a 2.5L bioreactor, which is the highest yield reported to date.
In the 30 L bioreactor, although the yield decreased slightly, it still reached 17.5 g/L.
These results indicate that through temperature-sensitive regulation strategy, metabolic flux optimization and fine adjustment of fermentation conditions, the high efficiency production of α-red myrrh by yeast fermentation and large-scale industrialization have a good prospect.
References: https://pubs.acs.org/doi/10.1021/acssynbio.4c00728