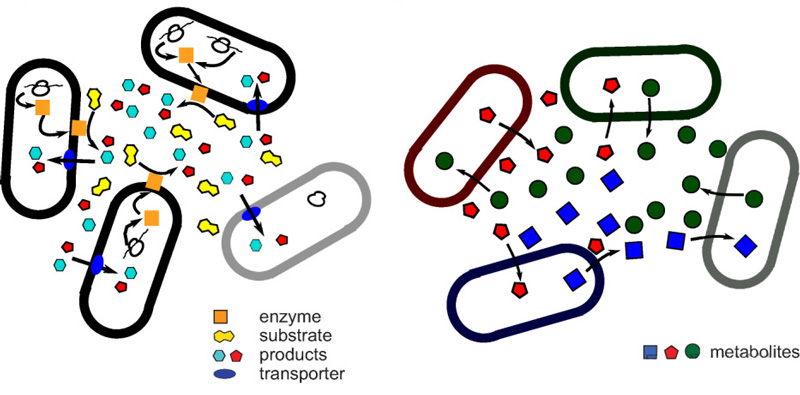
When a metabolic function is “leaky” to the community (i.e., partially available to other species), microbes can save energy by stopping production of that function and relying on other species to perform it.
Metabolic dependence in organisms can be formed by the loss of certain easily replaceable functions, such as the active deletion of certain genes.
The Black Queen hypothesis states that certain microbial genes encode extracellular products (such as metabolites or enzymes) that can be used by all or most microbes.
At this point, a species that has always been present in the community can give up the gene that codes for the synthetic product, choose to feed itself, and wait to be fed with metabolites produced by other members of the community.
In this case, mutations tend to wipe these genes out of the genome.

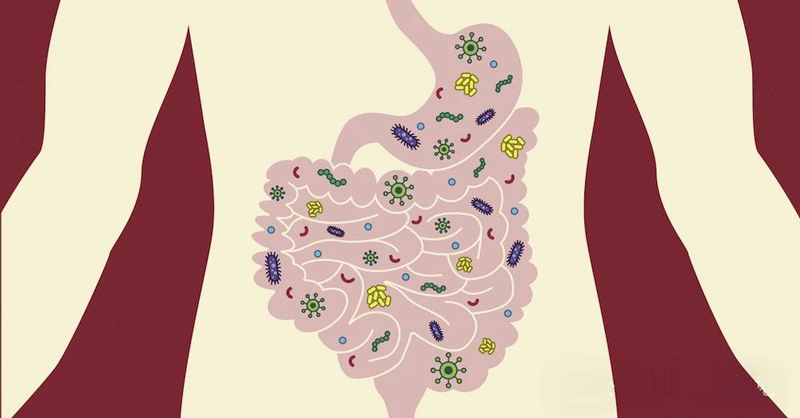
Whether host-related or not, the microbiome itself provides some strongly interdependent communities.
Microbial communities may contain thousands of interacting species, some of which may depend on others for survival.
For example, B vitamins are synthesized by only 40-65% of the human gut microbes.
The remaining 35-60% of gut microbes lack the B-vitamin synthesis pathway and therefore rely on B-vitamin synthesizing bacteria in the gut to survive.
Studies have shown that this interdependence is widespread in nature.
Among bacteria isolated from the human colon, mouth, soil and Marine ecosystems, there is a clear cross-feeding behavior, the exchange of metabolites between a bacterium and other microorganisms or macroorganisms.
Samples from different environments showed that the vast majority, perhaps as much as 99.9 percent, of bacterial species could not be cultured in the lab.
But in the presence of adjacent microorganisms or even adjacent secreted products, usual cell culture techniques can restore “unculturable” bacteria and increase the growth level of nutrient-deficient mutants.
This paints a typical picture of a highly cooperative microbial community in which some species contribute factors and enzymes necessary for growth for the benefit of their host or neighboring species.
However, this view often misunderstands the driving forces behind this dependency, putting a heavy artificial label on the phenomenon that makes it difficult to explain.
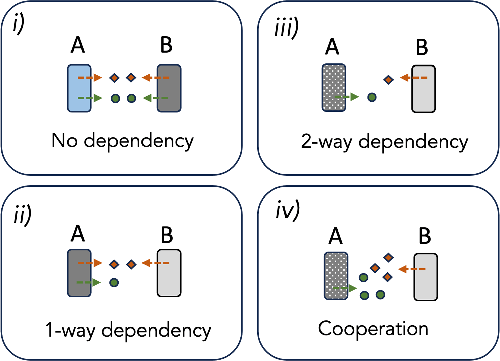
Traditionally, the scientific community has taken these dependencies for granted as “cooperation,” arguing that specific evolutionary mechanisms are needed to explain them.
In many cases, however, these features do not fall under the category of cooperation and do not require it to be explained, at least initially.
Because they are not selected for the benefits they bring to their partner species.
In contrast, the dependence of the microbiome may result from the streamlining of the genome, namely the adaptive gains made by the Black Queen dynamics.
At this point, the Black Queen hypothesis can be used to predict the direction of mutation in the microbial community, both which function of the deletion of genes is more conducive to survival, and the metabolic dependence caused by this mutation.
In a test experiment using two different E. coli ecotypes, E. coli were genetically engineered to feed on mutually exclusive resources (mannose and galactose) and thus occupied different ecological niches.
Both ecotypes contain ampicillin anti-plasmid (pBQ1), which contains a gene encoding beta-lactamase for the degradation of ampicillin outside the cell, a typical black queen function.
The team hypothesized that evolution should drive a race to the bottom, in which one ecotype would be transformed into a helper of ampicillin degradation, while the second ecotype would benefit by becoming antibiotic-sensitive.
The question is whether each ecological type will play the helper role about 50% of the time (i.e., randomly assigned), or whether one ecological type can predictably end up playing the helper role.

Experiments showed that in 15/18 repeated evolutionary populations, galactose metabolizers became sensitive to ampicillin and became dependent on ampicillin degradation services provided by mannose metabolizers.
However, mannose metabolizers retained antibiotic resistance throughout the experiment.
This is because mannose metabolizers have a lower intrinsic level of ampicillin resistance, so the benefit of expressing resistance genes is higher.
Thus, the higher opportunity cost experienced by one ecotype can predict which ecotype specializes in which role, even though the strains are genetically identical except for their niche related mutations.
In addition, the “silence is golden” trait of future beneficiaries regarding genes has also been theoretically demonstrated through agent-based modeling.
People often think, what do I deserve?
But in fact, this way of thinking may have to face two headaches, either unrealistic or difficult.
But if we see ourselves as tiny microbes in a sea of humanity, the problems we face may become clear and simple.
Now, try to ask yourself what I have that I can’t bear or no longer want to bear.
In the end, the opportunity cost will make the choice less difficult.