[Daily question] A detailed explanation of the binding mechanism of glutathione (GSH) and GST label protein
The specific binding mechanism of glutathione (GSH) to glutathione S-transferase (GST) tag protein involves multiple levels of molecular interactions, and the detailed mechanism is as follows:
Structural and functional background
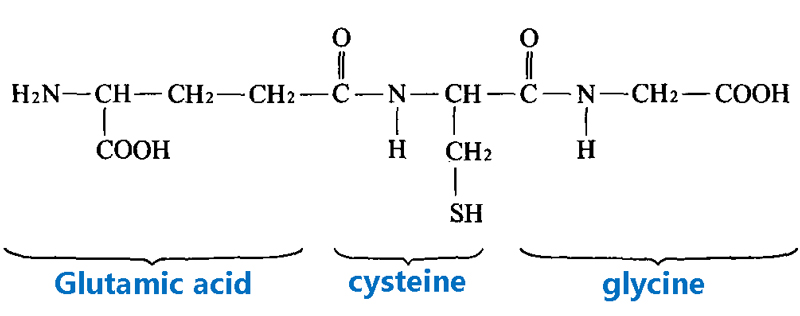
Glutathione (GSH)
A tripeptide consisting of glutamic acid, cysteine, and glycine with a cysteine residue containing a free sulfhydryl group (-SH).
GSH is not only an important antioxidant within cells, but also a natural substrate for GST.

GST tag protein
Glutathione S-Transferase is a widespread family of enzymes that catalyze the conjugation of the sulfhydryl group of GSH with electrophilic substrates (e.g. toxins, drugs).
In recombinant protein expression, GST is often used as an affinity tag for purification.
core of binding mechanism: interaction between GST and GSH
The combination of GST and GSH relies on the following key factors:
(1) Structural adaptation of active site
The active center of GST contains a highly conserved glutathione binding site (G-site) whose three-dimensional structure is complementary to specific regions of GSH through hydrogen bonding, hydrophobic interaction, and electrostatic attraction.
Recognition of sulfhydryl (-SH) : The sulfhydryl group of GSH forms a hydrogen bond network with tyrosine, serine or cysteine residues in G-site, while the surrounding hydrophobic environment stabilizes the positioning of sulfhydryl groups.
Adaptation of the tripeptide skeleton: the glutamate and glycine residues of GSH bind to the polar amino acids of GST (e.g., arginine, asparagine) by hydrogen bonding, respectively, while the hydrophobic side chains (e.g., the methyl group of cysteine) are embedded in the hydrophobic pocket.
(2) Dynamic combination process
Induced fit mechanism: After GSH enters the G-site, GST conformational changes (such as fine tuning of α helix or β fold) are triggered to further optimize the binding interface.
Synergistic binding effects: Some GST isoenzymes, such as GSTπ, require dimerization to form functional binding sites that enhance affinity with GSH.
Purification of GST tag protein
In biotechnology, the purification of GST tag proteins is based on the specific binding described above:
- Immobilized GSH matrix: GSH is covalently coupled to agarose resin to form an affinity chromatography medium.
- Binding stage: Proteins containing GST tags bind to immobilized GSH via the GST domain, and impurities are washed away.
- Competitive elution: Free GSH or reduced glutathione (e.g., 10-20 mM GSH) is added to compete for binding sites and release target proteins.
Key influencing factors
- Reducing state of the sulfhydryl group: The sulfhydryl group of GSH must be in the reducing state (-SH) to bind effectively, so a reducing agent (such as DTT or β-mercaptoethanol) needs to be added to the buffer.
- pH and ionic strength: Near-neutral conditions (pH 6.5-8.0) and appropriate ionic strength (e.g. 150 mM NaCl) maintain binding stability.
- Temperature: Usually performed at 4°C to reduce protease activity and non-specific binding.
Biological significance and extended application
Detoxification function: Natural GST binds and removes electrophilic toxins through GSH, protecting cells from oxidative damage.
Molecular tools: GST labeling systems are widely used in recombinant protein purification, protein interaction studies (such as GST pull-down experiments), and drug screening platforms.
Sum up
The binding of GSH and GST is a classic case of enzyme-substrate interaction, which combines structural adaptation, dynamic conformational change, and multiple non-covalent forces.
This property not only underpins the intracellular detoxification pathway, but also forms the basis for the design of efficient purification tools in biotechnology.